Pathogen-bound C-type lectin and ficolin enhance toll receptor signaling is activated through Spätzle1 cleavage in response to White Spot Syndrome Virus (WSSV) in Penaeus monodon
IF 3.9
2区 农林科学
Q1 FISHERIES
引用次数: 0
Abstract
The dynamic interaction between immune recognition molecules and signaling pathways in the innate immune response of Penaeus monodon to White Spot Syndrome Virus (WSSV) infection is unveiled in this study. Through comprehensive gene expression profiling, we demonstrate significant upregulation of key immune genes, including a specific C-type lectin and a defined ficolin isoform, in WSSV-infected hemocytes, underscoring their pivotal roles in pathogen recognition and antiviral defense. Leveraging advanced molecular techniques, we successfully expressed, purified, and characterized these recombinant proteins, revealing their time-dependent expression and high-affinity binding to lipopolysaccharides (LPS). Intriguingly, pathogen-bound lectin and ficolin were found to modulate Spätzle1 cleavage and enhance its interaction with a functionally characterized Toll receptor variant, suggesting a sophisticated mechanism for immune activation. Computational analyses using HADDOCK and PDBePISA further elucidated the structural and energetic basis of Toll receptor interactions with cleaved Spätzle1 peptides, highlighting the critical role of hydrophobic, electrostatic, and hydrogen bonding interactions in complex stabilization. Temporal expression dynamics of MyD88 and TRAF6 following Spätzle1 injection revealed a robust, time-dependent activation of Toll-mediated signaling pathways, with peak expression at 24 h post-injection. These findings not only deepen our understanding of crustacean immunity but also provide novel insights into the molecular mechanisms underlying pathogen recognition and immune modulation. The study positions this specific lectin and ficolin variants as key regulators of innate immunity, offering promising avenues for therapeutic interventions in aquaculture and beyond. By bridging molecular insights with functional validation, this work lays the groundwork for future research aimed at harnessing these immune molecules for sustainable disease management and immunotherapeutic applications.
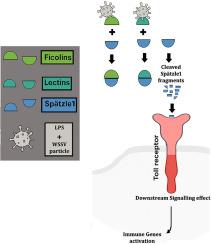
白斑综合征病毒(WSSV)对单对虾病原菌结合的c型凝集素和Ficolin通过Spätzle1裂解激活Toll受体信号。
本研究揭示了单对虾(Penaeus monodon)对白斑综合征病毒(White Spot Syndrome Virus, WSSV)感染的先天免疫应答中免疫识别分子与信号通路之间的动态相互作用。通过全面的基因表达谱分析,我们发现在感染wssv的血细胞中,关键免疫基因(包括一种特定的c型凝集素和一种确定的菲可林异构体)显著上调,强调了它们在病原体识别和抗病毒防御中的关键作用。利用先进的分子技术,我们成功地表达、纯化和表征了这些重组蛋白,揭示了它们的时间依赖性表达和与脂多糖(LPS)的高亲和力结合。有趣的是,病原体结合的凝集素和ficolin被发现调节Spätzle1的裂解,并增强其与功能特征的Toll受体变异的相互作用,这表明免疫激活的复杂机制。利用HADDOCK和PDBePISA的计算分析进一步阐明了Toll受体与裂解Spätzle1肽相互作用的结构和能量基础,强调了疏水、静电和氢键相互作用在络合物稳定中的关键作用。注射Spätzle1后,MyD88和TRAF6的时间表达动态显示,toll介导的信号通路具有强大的时间依赖性激活,在注射后24小时达到峰值表达。这些发现不仅加深了我们对甲壳类动物免疫的理解,而且为病原体识别和免疫调节的分子机制提供了新的见解。该研究将这种特定的凝集素和菲可林变体定位为先天免疫的关键调节因子,为水产养殖及其他领域的治疗干预提供了有希望的途径。通过将分子见解与功能验证相结合,这项工作为未来的研究奠定了基础,旨在利用这些免疫分子进行可持续的疾病管理和免疫治疗应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fish & shellfish immunology
农林科学-海洋与淡水生物学
CiteScore
7.50
自引率
19.10%
发文量
750
审稿时长
68 days
期刊介绍:
Fish and Shellfish Immunology rapidly publishes high-quality, peer-refereed contributions in the expanding fields of fish and shellfish immunology. It presents studies on the basic mechanisms of both the specific and non-specific defense systems, the cells, tissues, and humoral factors involved, their dependence on environmental and intrinsic factors, response to pathogens, response to vaccination, and applied studies on the development of specific vaccines for use in the aquaculture industry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


