A pathogen peptidoglycan scaffold coated with artificial biomembrane promotes broad resistance to bacterial infections by dynamically reprogramming macrophage metabolism
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
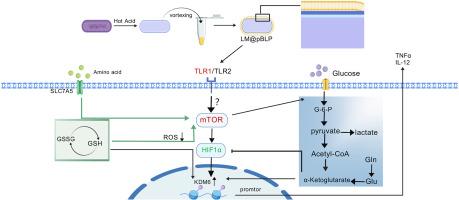
The increasing severity of multidrug-resistant (MDR) bacteria and the shortage of effective treatment strategies urgently require the development of new immunotherapies to combat superbug infections. Trained immunity may offer a novel and effective mechanism to combat resistant superbugs. However, there are currently few materials capable of effectively activating trained immunity, highlighting the need for new agents that provide more durable protection. In this study, we developed a bacterium-like particle (BLP) based on protein-free artificial biomembrane coating immune activator, named LM@pBLP, which features a simple and rapid preparation process, excellent biocompatibility, long-term stability, and a cost-effective advantage. LM@pBLP trains the immune system to target a broad range of pathogens, offering rapid, broad-spectrum, and long-lasting protection against MDR infections. After stimulation with LM@pBLP, it activates glutathione metabolism and amino acid metabolism, induces macrophage metabolic and epigenetic reprogramming changes, and regulates phagocytosis and inflammatory responses to infection. Additionally, LM@pBLP regulates reactive oxygen species (ROS), thereby maintaining oxidative stress homeostasis. Our study demonstrates that LM@pBLP primarily provides rapid, broad-spectrum, and long-lasting protection for experimental animals by activating trained immunity, which opens a new avenue for addressing MDR infections.
一种被人工生物膜包裹的病原体肽聚糖支架通过动态重编程巨噬细胞代谢促进对细菌感染的广泛抵抗
随着耐多药(MDR)细菌的严重程度日益增加,以及有效治疗策略的缺乏,迫切需要开发新的免疫疗法来对抗超级细菌感染。训练有素的免疫系统可能提供一种新的有效机制来对抗耐药的超级细菌。然而,目前很少有材料能够有效地激活经过训练的免疫,这突出表明需要提供更持久保护的新制剂。在本研究中,我们开发了一种基于无蛋白人工生物膜涂层的细菌样颗粒(BLP)免疫激活剂,命名为LM@pBLP,其制备工艺简单快速,具有良好的生物相容性,长期稳定性和成本效益优势。LM@pBLP训练免疫系统以广泛的病原体为目标,提供快速、广谱和持久的耐多药感染保护。LM@pBLP刺激后,激活谷胱甘肽代谢和氨基酸代谢,诱导巨噬细胞代谢和表观遗传重编程改变,调节吞噬和对感染的炎症反应。此外,LM@pBLP调节活性氧(ROS),从而维持氧化应激稳态。我们的研究表明,LM@pBLP主要通过激活训练免疫为实验动物提供快速、广谱和持久的保护,这为解决耐多药感染开辟了一条新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


