Incorporating silicon-doped carbon dots into europium chelated silica microspheres for the ratiometric fluorescent and colorimetric detection of Fe3+ ions
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
In this study, a novel hybrid luminescent microsphere, Si-CDs@SiO2-Eu was designed and successfully synthesized by encapsulating blue-emitting silicon-doped carbon quantum dots (Si-CDs) into carboxyl-modified SiO2 microsphere and coordinating with Eu3+ ions. The SiO2 matrix provides a stable dispersion environment for Si-CDs and serves as an effective platform for anchoring Eu3+ on the surface of microsphere, thereby realizing the spatial isolation and establishing dual-emission characteristic. The dual-emission microspheres can be served as a ratiometric fluorescent probe for the detection of Fe3+ in water with the red emission of Eu3+ ion as response and the blue emission of Si-CDs as reference. It displays high selectivity and sensitivity for Fe3+ with a detection limit of 1.41 μM. It is worth noting that an easy-to-differentiate color change for visual detection is also observed. In addition, the luminescence quenching mechanism study reveals that absorption competition quenching (ACQ) mechanism and cation exchange cause luminescence quenching at 617 nm, but have little effect at 400 nm, thus realizing the fluorescent ratiometric detection of Fe3+. Si-CDs@SiO₂-Eu functions as a dual-mode probe that integrates ratiometric (quantitative) and colorimetric (qualitative) detection, enabling real-time monitoring of Fe3+ and enhancing detection reliability.
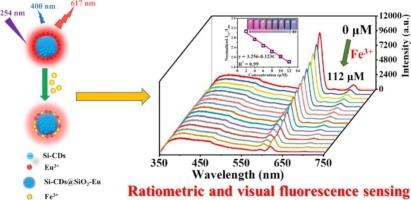
将掺硅碳点掺入铕螯合二氧化硅微球中,用于比例荧光和比色法检测Fe3+离子
本研究设计并成功合成了一种新型的杂化发光微球Si-CDs@SiO2-Eu,该微球是将蓝色硅掺杂碳量子点(Si-CDs)封装在羧基修饰的SiO2微球中,并与Eu3+离子配位。SiO2基体为Si-CDs提供了稳定的色散环境,并为Eu3+在微球表面的锚定提供了有效的平台,从而实现了空间隔离,建立了双发射特性。该双发射微球可作为比例荧光探针,以Eu3+离子的红色发射为响应,Si-CDs的蓝色发射为参比,用于水中Fe3+的检测。对Fe3+具有较高的选择性和灵敏度,检测限为1.41 μM。值得注意的是,还观察到一种易于区分的视觉检测颜色变化。此外,发光猝灭机理研究表明,吸收竞争猝灭(ACQ)机制和阳离子交换导致617 nm处的发光猝灭,而在400 nm处影响不大,从而实现了Fe3+的荧光比例检测。Si-CDs@SiO₂-Eu是一种双模探针,集成了比率(定量)和比色(定性)检测,可以实时监测Fe3+,提高检测可靠性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


