An injectable phosphocreatine-grafted hydrogel incorporating hierarchically structured teriparatide/SrZnP-functionalized Zn–Cu particles for osteogenesis–angiogenesis coupling and osteoporotic bone regeneration
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Osteoporotic bone defects remain a major clinical challenge due to impaired osteogenesis, insufficient angiogenesis, excessive osteoclast activity, and increased susceptibility to infection. To address these issues, we developed an injectable phosphocreatine-grafted gelatin hydrogel (GGP) incorporating hierarchically structured Zn-Cu particles functionalized with a teriparatide (PTH)/strontium–zinc phosphate (SrZnP) hybrid coating. This multifunctional hydrogel was fabricated via enzymatic and ionic coordination crosslinking, yielding improved mechanical properties and sustained release of Zn2+, Sr2+, and PTH. In vitro evaluations demonstrated that the hydrogel enhanced BMSC proliferation, osteogenic differentiation, and mineralization, promoted HUVEC migration, tube formation, and angiogenic marker expression, and simultaneously inhibited osteoclastogenesis and bacterial growth. Transcriptomic analysis and inhibitor experiments revealed a dual paracrine mechanism mediating bone–vascular coupling: BMSC-derived HIF-1α–VEGF signaling facilitated angiogenesis, while HUVEC-derived PI3K–Akt–BMP-2 signaling enhanced osteogenesis. In vivo, the PTH/SrZnP@ZnCu-GGP hydrogel significantly accelerated bone regeneration and neovascularization in an ovariectomized rat calvarial defect model, accompanied by upregulated expression of BMP-2, RUNX2, p-Akt, and CD31. Collectively, this injectable hydrogel system offers a robust and translationally feasible strategy for coordinated osteogenesis–angiogenesis coupling, osteoclast suppression, and antibacterial defense, thus holding strong potential for the regeneration of osteoporotic bone defects.
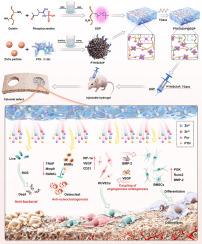
一种可注射的磷酸肌酸移植水凝胶,含有分层结构的三立帕肽/ srznp功能化的锌- cu颗粒,用于骨生成-血管生成耦合和骨质疏松性骨再生
骨质疏松性骨缺损由于成骨功能受损、血管生成不足、破骨细胞活性过高以及对感染的易感性增加,仍然是一个主要的临床挑战。为了解决这些问题,我们开发了一种可注射的磷酸肌酸接枝明胶水凝胶(GGP),其中包含了层阶结构的锌-铜颗粒,这些颗粒被三立帕肽(PTH)/磷酸锶锌(SrZnP)杂化涂层功能化。该多功能水凝胶是通过酶和离子配位交联制备的,具有更好的力学性能和Zn2+、Sr2+和PTH的缓释。体外实验表明,水凝胶增强BMSC增殖、成骨分化和矿化,促进HUVEC迁移、管形成和血管生成标志物表达,同时抑制破骨细胞生成和细菌生长。转录组学分析和抑制剂实验揭示了介导骨-血管耦合的双重旁分泌机制:bmsc衍生的HIF-1α-VEGF信号促进血管生成,而huvec衍生的PI3K-Akt-BMP-2信号促进骨生成。在体内,PTH/SrZnP@ZnCu-GGP水凝胶显著促进去卵巢大鼠颅骨缺损模型的骨再生和新生血管形成,同时上调BMP-2、RUNX2、p-Akt和CD31的表达。总的来说,这种可注射的水凝胶系统为骨生成-血管生成耦合、破骨细胞抑制和抗菌防御提供了一种强大的、翻译上可行的策略,因此在骨质疏松性骨缺陷的再生方面具有强大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


