A review on structural modifications of parthenin extracted from Parthenium hysterophorus L
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Parthenin is a sesquiterpene lactone extracted from Parthenium hysterophorus that possesses a broad spectrum of biological activities, including anti-inflammatory, antimicrobial, anticancer, and allelopathic properties. However, its clinical applications are hindered by cytotoxicity, poor solubility, low bioavailability, and instability in physiological conditions. To address these challenges, numerous structural modifications have been explored to improve its pharmacokinetic properties while enhancing its therapeutic potential. This review provides a comprehensive overview of the chemical modifications of parthenin, including hydroxylation, halogenation, Michael addition, and lactone ring alterations. Modifications, particularly in the lactone and cyclopentenone rings, can significantly enhance its anticancer, antimicrobial, and anti-inflammatory activities while reducing toxicity. The review also highlights recent pharmacological advancements and outlines promising directions for future drug development based on the parthenin derivatives. Overall, the study underscores the value of parthenin and its derivatives as innovative therapeutic agents and contributes to the progress of natural product-based drug design.
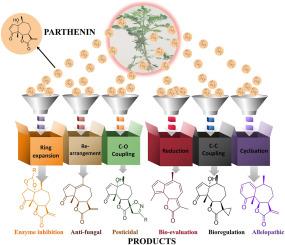
帕提宁提取物的结构修饰研究进展
帕thenin是一种从帕thenium hysterophorus中提取的倍半萜内酯,具有广泛的生物活性,包括抗炎、抗菌、抗癌和化感作用。然而,其临床应用受到细胞毒性、溶解度差、生物利用度低和生理条件不稳定等因素的阻碍。为了应对这些挑战,已经探索了许多结构修饰,以改善其药代动力学特性,同时增强其治疗潜力。本文综述了parthenin的化学修饰,包括羟基化、卤化、Michael加成和内酯环改变。修饰,特别是内酯和环戊酮环的修饰,可以显著增强其抗癌、抗菌和抗炎活性,同时降低毒性。综述还强调了最近的药理学进展,并概述了未来基于parthenin衍生物的药物开发的有希望的方向。总的来说,该研究强调了parthenin及其衍生物作为创新治疗药物的价值,并有助于基于天然产物的药物设计的进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


