FeCl3/I2-catalyzed tandem aerobic oxidative annulation of α-aminoketone hydrochlorides with aldehydes: synthesis of substituted oxazoles
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A facile and efficient method for the synthesis of oxazoles has been developed through FeCl3/I2-catalyzed tandem aerobic oxidative annulation of α-aminoketone hydrochlorides with aldehydes. The reaction employs commercially available α-aminoketone hydrochlorides and aldehydes as the starting materials, offering notable advantages including operational simplicity, one-pot synthesis, low cost, broad scope, and good functional group tolerance. The synthetic utility has been demonstrated by the syntheses of 33 examples of oxazoles in yields up to 92 %. Furthermore, the protocol was successfully applied to gram-scale synthesis and to the one-step synthesis of the natural product oxytrofalcatin C.
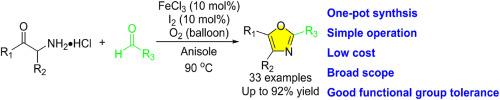
FeCl3/ i2催化α-氨基酮与醛的串联好氧环化反应:取代恶唑的合成
通过FeCl3/ i2催化α-氨基酮与醛的串联好氧环化反应,建立了一种简便、高效的合成恶唑的方法。该反应以市售的α-氨基酮类盐酸和醛为原料,具有操作简单、一锅合成、成本低、反应范围广、官能团耐受性好等优点。合成了33个恶唑,产率高达92%,证明了该合成方法的实用性。此外,该方案还成功地应用于克级合成和一步合成天然产物氧化镰镰素C。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


