Failure mechanism analysis for AMPS-family polymers in high-salinity hydrothermal environments
IF 7.4
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Polymers derived from 2-acrylamido-2-methylpropane sulfonic acid (AMPS) are extensively employed as chemical additives in oil and gas drilling operations. However, as drilling increasingly targets deep and ultra-deep reservoirs, AMPS-family polymers also undergo functional degradation as the extreme downhole conditions of high salinity (up to saturation), high pressure (45–150 MPa), and high temperature (150–250 °C). This review is the first to focus on the special topic, i.e., failure mechanism of AMPS-family polymers in high-salinity hydrothermal environments. More than ten chemical groups have been identified from 36 monomers that are commonly used for synthesizing AMPS-family polymers. Their polymer functions, degradation mechanisms and improvement methods are discussed in details combining with the physicochemical properties of high-salinity subcritical water. Acid-catalyzed hydrolysis is identified as the predominant degradation mechanism. The hydrothermal stability of functional groups follows the order: benzene > organosilicon > quaternary ammonium > sulfonic acid > heterocycle > amide > carboxylic acid > ether > alcohol > ester. Key strategies for enhancing stability include controlling drilling mud pH and increasing polymer steric hindrance. Future fundamental research is recommended to focus on inorganic salt behavior in complex subcritical water systems, hydrothermal reaction pathways of relevant monomers and polymers, and the development of next-generation polymers incorporating novel functional groups. Information provided by this review can promote a better understanding of polymer behaviors in high-salinity hydrothermal environments, a guideline for selecting suitable monomers, and recommendations for new polymer development.
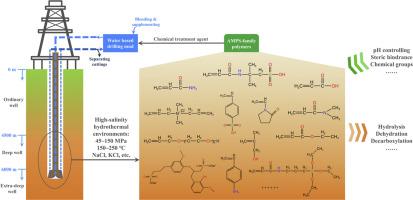
amps家族聚合物在高盐度水热环境中的破坏机理分析
由2-丙烯酰胺-2-甲基丙烷磺酸(AMPS)衍生的聚合物被广泛用作石油和天然气钻井作业中的化学添加剂。然而,随着钻井越来越多地瞄准深层和超深层油藏,amps家族聚合物也会在高盐度(达到饱和)、高压(45-150 MPa)和高温(150-250°C)的极端井下条件下发生功能降解。本文首次对amps家族聚合物在高盐度水热环境中的破坏机制进行了专题综述。从36个通常用于合成amps家族聚合物的单体中鉴定出10多个化学基团。结合高盐度亚临界水的理化性质,详细讨论了它们的聚合物功能、降解机理和改进方法。酸催化水解被认为是主要的降解机制。官能团的水热稳定性顺序为:苯>;有机硅>;季铵>;磺酸>;杂环>;酰胺>;羧酸>;醚>;醇>;酯。提高稳定性的关键策略包括控制钻井液pH值和增加聚合物的位阻。建议未来的基础研究重点放在复杂亚临界水体系中无机盐的行为,相关单体和聚合物的水热反应途径,以及含有新官能团的下一代聚合物的开发上。本综述提供的信息有助于更好地了解聚合物在高盐度水热环境中的行为,为选择合适的单体提供指导,并为新聚合物的开发提供建议。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Degradation and Stability
化学-高分子科学
CiteScore
10.10
自引率
10.20%
发文量
325
审稿时长
23 days
期刊介绍:
Polymer Degradation and Stability deals with the degradation reactions and their control which are a major preoccupation of practitioners of the many and diverse aspects of modern polymer technology.
Deteriorative reactions occur during processing, when polymers are subjected to heat, oxygen and mechanical stress, and during the useful life of the materials when oxygen and sunlight are the most important degradative agencies. In more specialised applications, degradation may be induced by high energy radiation, ozone, atmospheric pollutants, mechanical stress, biological action, hydrolysis and many other influences. The mechanisms of these reactions and stabilisation processes must be understood if the technology and application of polymers are to continue to advance. The reporting of investigations of this kind is therefore a major function of this journal.
However there are also new developments in polymer technology in which degradation processes find positive applications. For example, photodegradable plastics are now available, the recycling of polymeric products will become increasingly important, degradation and combustion studies are involved in the definition of the fire hazards which are associated with polymeric materials and the microelectronics industry is vitally dependent upon polymer degradation in the manufacture of its circuitry. Polymer properties may also be improved by processes like curing and grafting, the chemistry of which can be closely related to that which causes physical deterioration in other circumstances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


