Thermally activating IrCoP@CNT: A double-phase synergistic catalyst for ultra-efficient acidic oxygen evolution and sustainable Lead electrodeposition
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The development of high-performance acidic oxygen evolution reaction (OER) catalysts with ultralow overpotential is crucial for green electrolysis, particularly to address the competitive anodic oxidation between water molecules and Pb2+ during lead recovery. In this work, we proposed IrCoP@CNT through an in-situ growth of ZIF-67 on carbon nanotubes (CNTs), controlled phosphidation, and precise deposition of iridium (Ir) species. The superior architecture integrates highly dispersed, nanoscale metallic Ir and crystalline IrP2 active sites intimately anchored within a three-dimensional conductive network formed by CNTs intertwined with ZIF-derived N, P-codoped carbon, facilitating exceptional charge/mass transport, and enhances structural robustness. Remarkably, IrCoP@CNT achieves an ultralow OER overpotential of 113 mV (10 mA cm−2) in 1 M methanesulfonic acid (MSA) at 90 °C, while completely suppressing parasitic Pb2+ oxidation to PbO2. This exceptional selectivity enables near-quantitative lead deposition (99.87 % current efficiency) with record-low energy consumption (420.68 kWh t−1), overcoming the OER/Pb2+ oxidation competition. IrCoP@CNT establishes a new paradigm, uniquely merging ultra-high intrinsic OER kinetics with the fundamental elimination of competing anodic side reactions, dramatically minimizing energy dissipation and paving the way for highly efficient and sustainable electrochemical metal recycling.
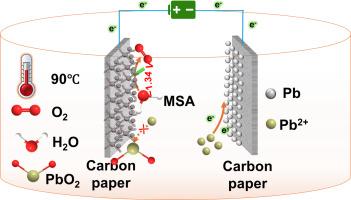
热活化IrCoP@CNT:一种用于超高效酸性析氧和可持续铅电沉积的双相协同催化剂
开发高性能的超低过电位酸性析氧反应(OER)催化剂对于绿色电解至关重要,特别是对于解决铅回收过程中水分子与Pb2+之间的竞争性阳极氧化问题。在这项工作中,我们通过在碳纳米管(CNTs)上原位生长ZIF-67、控制磷化和精确沉积铱(Ir)来提出IrCoP@CNT。优越的结构将高度分散的纳米级金属Ir和晶体IrP2活性位点紧密地固定在由CNTs与zif衍生的N, p共掺杂碳交织形成的三维导电网络中,促进了特殊的电荷/质量传输,并增强了结构的稳健性。值得注意的是,IrCoP@CNT在1 M甲基磺酸(MSA)中在90°C下实现了113 mV (10 mA cm−2)的超低OER过电位,同时完全抑制了寄生Pb2+氧化为PbO2。这种特殊的选择性使得铅沉积接近定量(99.87%电流效率),能耗低(420.68 kWh t - 1),克服了OER/Pb2+氧化的竞争。IrCoP@CNT建立了一个新的范例,独特地将超高固有OER动力学与基本消除竞争性阳极副反应相结合,极大地减少了能量消耗,为高效和可持续的电化学金属回收铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


