Photochemical transformation of dichloromethane to chloroform: insights into the mechanism of aza[5]helicene photoprotonation
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-09-08
DOI:10.1016/j.jphotochem.2025.116767
引用次数: 0
Abstract
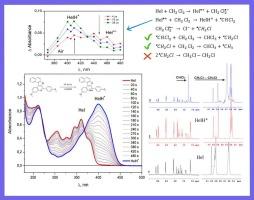
Aza[5]helicenes of the furoquinoline series have two important features: (a) possibility to modulate photophysical properties by protonation, and (b) they are good fluorophores that exhibit exceptional photostability in chlorine-free organic solvents. Light irradiation in chlorine-containing solvents was known to result in photoprotonation. In this work photophysics and photochemistry of a typical aza[5]helicene, namely 3-methoxy-6-(4-methoxyphenyl)naphtho[1′,2′:4,5]furo[2,3-c]quinoline (compound Hel) in dichloromethane (DCM) was studied using stationary photolysis with UV–Vis and NMR registration, time-resolved fluorescence and nanosecond laser flash photolysis. The free base aza[5]helicene was found for the first time to promote the transformation of dichloromethane to chloroform upon irradiation with UV light (308 nm). Studies of the quantitative characteristics of Hel and HelH+ (fluorescence quantum yields, spectra and kinetic properties of the triplet states –showed that photoprotonation occurs in two ways. The first pathway is started by an electron transfer from an excited Hel molecule to a solvent molecule, followed by an H atom transfer from a solvent molecule to the Hel![]() + radical cation. The second pathway of HelH+ formation is the reaction between Hel and HCl formed at the first stage. A quantitative mechanism of photoprotonation is proposed that explains all the observed experimental data.
+ radical cation. The second pathway of HelH+ formation is the reaction between Hel and HCl formed at the first stage. A quantitative mechanism of photoprotonation is proposed that explains all the observed experimental data.
二氯甲烷光化学转化为氯仿:aza b[5]螺旋烃光质子化机理的研究
呋喃喹啉系列的Aza b[5]螺旋烯具有两个重要特征:(a)可能通过质子化来调节光物理性质;(b)它们是良好的荧光团,在无氯有机溶剂中表现出优异的光稳定性。已知光照射在含氯溶剂中会引起光质子化。本文采用紫外-可见和核磁共振配准、时间分辨荧光和纳秒激光闪光光解等固定光解方法,研究了典型的含氮[5]螺旋烯3-甲氧基-6-(4-甲氧基苯基)萘[1′,2′:4,5]呋喃[2,3-c]喹啉(化合物Hel)在二氯甲烷(DCM)中的光物理和光化学性质。在308 nm紫外光照射下,首次发现了促进二氯甲烷转化为氯仿的游离碱aza -[5]螺旋烯。对Hel和HelH+的定量特征(荧光量子产率、光谱和三态动力学性质)的研究表明,光质子化以两种方式发生。第一种途径是由电子从受激发的Hel分子转移到溶剂分子,然后是H原子从溶剂分子转移到Hel+自由基阳离子。形成HelH+的第二种途径是第一阶段形成的Hel和HCl之间的反应。提出了光质子化的定量机理,解释了所有观察到的实验数据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


