Adsorption mechanism of phosphate and arsenate in water by Al-doped Zr-based MOF and theoretical DFT study
IF 6.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Excessive phosphorus and arsenic in water bodies not only destroy ecosystems but also pose a serious threat to human health. In this study, a series of Al-doped modified metal-organic frameworks (Zr-Al-MOF) were prepared by solvothermal method, which achieved efficient removal of phosphate and arsenate in water. Due to the use of inexpensive Al salts, the material has a lower cost and is more economical. The molar ratio of metal salts, adsorption time, solution pH, initial concentration, temperature and coexisting anions were studied, and it was found that when the molar ratio of Zr: Al was 2, Zr-Al-MOF had the best adsorption performance for phosphate and arsenate, and the maximum adsorption capacity was 93.04 mg P/g and 173.83 mg As/g, respectively. It traps phosphate and arsenate at a fast reaction rate and can be recycled repeatedly. In addition, 0.15 g/L of 2Zr-Al-MOF can effectively reduce the phosphate and arsenate content in the contaminated spring water samples of Yangzonghai Lake to the standard range of drinking water, which further confirms the application potential of 2Zr-Al-MOF. By FT-IR and XPS analysis, it was found that the adsorption mechanism was ligand exchange, electrostatic attraction and hydrogen bond formation. The theoretical calculation shows that the adsorption energy is negative, which indicates that 2Zr-Al-MOF is attractive to phosphate and arsenate, and the adsorption state is stable. The results show that 2Zr-Al-MOF is an effective phosphate and arsenate adsorbent and has broad application prospects in eutrophication water treatment.
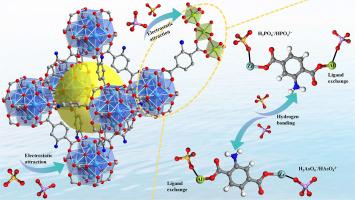
掺铝zr基MOF对水中磷酸盐和砷酸盐的吸附机理及理论DFT研究
水体中磷、砷超标不仅破坏生态系统,而且对人体健康构成严重威胁。本研究采用溶剂热法制备了一系列掺铝改性金属有机骨架(Zr-Al-MOF),实现了对水中磷酸盐和砷酸盐的高效脱除。由于使用了廉价的铝盐,该材料成本更低,更经济。研究了金属盐的摩尔比、吸附时间、溶液pH、初始浓度、温度和共存阴离子等因素,发现当Zr: Al的摩尔比为2时,Zr-Al- mof对磷酸盐和砷酸盐的吸附性能最好,最大吸附量分别为93.04 mg P/g和173.83 mg As/g。它以快速的反应速率捕获磷酸盐和砷酸盐,并可重复回收。此外,0.15 g/L的2Zr-Al-MOF能有效降低阳宗海污染泉水样品中磷酸盐和砷酸盐含量至饮用水标准范围,进一步证实了2Zr-Al-MOF的应用潜力。通过FT-IR和XPS分析,发现其吸附机理为配体交换、静电吸引和氢键形成。理论计算表明,吸附能为负,说明2Zr-Al-MOF对磷酸盐和砷酸盐具有吸引力,吸附状态稳定。结果表明,2Zr-Al-MOF是一种有效的磷酸盐和砷酸盐吸附剂,在富营养化水处理中具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Environmental Sciences-china
环境科学-环境科学
CiteScore
13.70
自引率
0.00%
发文量
6354
审稿时长
2.6 months
期刊介绍:
The Journal of Environmental Sciences is an international journal started in 1989. The journal is devoted to publish original, peer-reviewed research papers on main aspects of environmental sciences, such as environmental chemistry, environmental biology, ecology, geosciences and environmental physics. Appropriate subjects include basic and applied research on atmospheric, terrestrial and aquatic environments, pollution control and abatement technology, conservation of natural resources, environmental health and toxicology. Announcements of international environmental science meetings and other recent information are also included.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


