Horizontally transferred NADAR genes contribute to immune defense of ladybird beetles against bacterial infection
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Horizontal gene transfer (HGT) is now widely recognized as an important mechanism contributing to host immunity and adaptation. Ladybird beetles, with their diverse diets and habitats, encounter a broad spectrum of microbial threats, making effective immune responses critical for their survival. However, the immune roles of HGT-acquired genes in ladybirds remain largely unexplored. To address this gap, we investigated HGT of a NADAR (NAD- and ADP-ribose-associated) domain-containing gene from microorganisms to insects. Phylogenetic analyses revealed that NADAR genes in ladybird beetles form a well-supported clade nested within a larger group composed primarily of bacterial sequences, providing strong evidence for an HGT origin. Sampling across 69 ladybird species suggests that NADAR genes originated in the Coccinellidae family and were subsequently retained or duplicated across ladybird genomes, indicating their functional importance. Using the ladybird Cryptolaemus montrouzieri as a model, we observed that the expression levels of CmNADAR1 and CmNADAR2 were significantly upregulated in response to bacterial infection. Immune challenges combined with RNA interference targeting NADAR genes led to reduced survival rates and marked necrosis in intestinal tissues, compared to controls exposed to either bacterial infection or dsRNA alone. Together, our results demonstrate that NADAR genes in ladybird beetles were acquired through horizontal gene transfer and contribute to immune defense against bacterial infection.
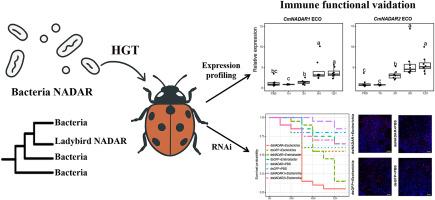
水平转移的NADAR基因有助于瓢虫抵抗细菌感染的免疫防御
水平基因转移(HGT)被广泛认为是促进宿主免疫和适应的重要机制。瓢虫有着多样化的饮食和栖息地,面临着广泛的微生物威胁,因此有效的免疫反应对它们的生存至关重要。然而,hgt获得性基因在瓢虫中的免疫作用在很大程度上仍未被探索。为了解决这一空白,我们研究了微生物和昆虫中含有NADAR (NAD-和adp -核糖相关)结构域基因的HGT。系统发育分析显示,瓢虫的NADAR基因在一个主要由细菌序列组成的更大的群体中形成了一个很好的支持分支,为HGT起源提供了有力的证据。对69种瓢虫的抽样表明,NADAR基因起源于瓢虫科,随后在瓢虫基因组中保留或复制,表明其功能重要性。以montrouzieri隐虫瓢虫为模型,我们观察到CmNADAR1和CmNADAR2的表达水平在对细菌感染的反应中显著上调。与单独暴露于细菌感染或dsRNA的对照组相比,免疫挑战结合靶向NADAR基因的RNA干扰导致肠道组织存活率降低和明显坏死。总之,我们的研究结果表明,瓢虫的NADAR基因是通过水平基因转移获得的,并有助于免疫防御细菌感染。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


