Conjugated bile acid-driven CD14+CD16+ monocyte infiltration promotes cholestatic liver injury by enhancing hepatocyte necroptosis
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
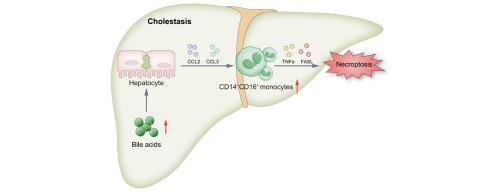
Cell death and inflammation cause liver injury during cholestasis. Monocyte infiltration is crucial for the inflammatory response in tissues and organs. Thus, to study the role of monocytes in cholestatic liver disease, we used single-cell RNA sequencing (scRNA-seq) to analyze liver non-parenchymal cells (NPCs) from patients with obstructive cholestasis (OC).
Methods
Hepatic monocyte infiltration in liver was assessed using scRNA-seq and multiplex immunofluorescence. In vivo functional validation was conducted through serum biochemistry, liver histopathology, RT-qPCR, and Western blotting after intermediate monocyte infusion and elimination in mice. In vitro, CD14+CD16+ monocytes were isolated by flow cytometry for analysis of tumor necrosis factor-alpha (TNFα) and factor-related apoptosis ligand (FASL) expression.
Results
scRNA-seq and multiplex immunofluorescence revealed that CD14+CD16+ monocyte infiltration was increased in OC livers (n = 5–20; p <0.0001). The number of CD14+CD16+ monocytes was positively correlated with liver injury severity (alanine aminotransferase (ALT), n = 20, p = 0.00002; aspartate aminotransferase (AST), n = 20, p = 0.002). Infusion of human CD14+CD16+ monocytes (i.e. human intermediate monocytes) exacerbated cholestatic liver injury in mice fed 1% lithocholic acid (ALT, n = 5, p <0.01; AST, n = 5, p <0.01). Meanwhile, mice that received mouse intermediate monocytes also exhibited higher cholestatic liver injury (ALT, n = 5, p <0.01; AST, n = 5, p <0.01). Mechanistically, CD14+CD16+ monocyte infiltration increased hepatic levels of TNFα (n = 5, p <0.01) and FASL (n = 5, p <0.01), activating the receptor-interacting serine/threonine-protein kinase (RIPK)-1–RIPK3–mixed-lineage kinase domain-like protein (MLKL) axis to promote hepatocyte necroptosis. CD14+CD16+ monocytes isolated from peripheral blood mononuclear cells (PBMCs) from patients with OC expressed higher levels of TNFα (n = 3, p <0.05) and FASL (n = 3, p <0.05) compared with other PBMC populations. Bile acid-stressed hepatocytes drove CD14+CD16+ monocyte infiltration via JNK/c-Jun-mediated CCL2 (n = 5-20, p <0.0001) and CCL3 (n = 5-20, p <0.05) upregulation.
Conclusion
Infiltrated CD14+CD16+ monocytes promote hepatocyte necroptosis and exacerbate cholestatic liver injury. Thus, targeting their chemotaxis represents a promising therapeutic strategy against cholestasis.
Impact and implications
This study revealed that CD14+CD16+ monocyte-derived tumor necrosis factor-alpha (TNFα) and factor-related apoptosis ligand (FASL) drive hepatocyte necroptosis via the receptor-interacting serine/threonine-protein kinase (RIPK)-1–RIPK3–mixed-lineage kinase domain-like protein (MLKL) axis, establishing these infiltrating monocytes as crucial mediators of cholestatic liver injury. Bile acid-stressed hepatocytes promoted CD14+CD16+ monocyte infiltration by upregulating CCL2 and CCL3 via JNK/c-Jun signaling. These findings highlight the therapeutic potential of modulating monocyte infiltration or necroptosis pathways to alleviate disease progression, offering novel strategies for treating OC and related liver pathologies.
偶联胆汁酸驱动CD14+CD16+单核细胞浸润通过增强肝细胞坏死而促进胆汁淤积性肝损伤
背景:胆汁淤积时,细胞死亡和炎症可引起肝损伤。单核细胞浸润对组织和器官的炎症反应至关重要。因此,为了研究单核细胞在胆汁淤积性肝病中的作用,我们使用单细胞RNA测序(scRNA-seq)分析梗阻性胆汁淤积(OC)患者的肝脏非实质细胞(npc)。方法采用scRNA-seq法和多重免疫荧光法检测肝组织单核细胞浸润情况。通过小鼠中间单核细胞输注和消除后的血清生化、肝脏组织病理学、RT-qPCR和Western blotting进行体内功能验证。在体外,流式细胞术分离CD14+CD16+单核细胞,分析肿瘤坏死因子- α (tnf - α)和因子相关凋亡配体(FASL)的表达。结果scrna -seq和多重免疫荧光显示,肝癌组织中CD14+CD16+单核细胞浸润增加(n = 5 ~ 20; p <0.0001)。CD14+CD16+单核细胞数量与肝损伤严重程度呈正相关(丙氨酸转氨酶(ALT), n = 20, p = 0.00002;谷草转氨酶(AST), n = 20, p = 0.002)。输注人CD14+CD16+单核细胞(即人中间单核细胞)加重1%石胆酸喂养小鼠的胆汁沉积性肝损伤(ALT, n = 5, p <0.01; AST, n = 5, p <0.01)。同时,给予小鼠中间单核细胞的小鼠也表现出较高的胆汁沉积性肝损伤(ALT, n = 5, p <0.01; AST, n = 5, p <0.01)。机制上,CD14+CD16+单核细胞浸润增加肝脏TNFα (n = 5, p <0.01)和FASL (n = 5, p <0.01)水平,激活受体相互作用的丝氨酸/苏氨酸蛋白激酶(RIPK)-1 - ripk3混合谱系激酶结构域样蛋白(MLKL)轴,促进肝细胞坏死。从OC患者外周血单核细胞(PBMCs)中分离的CD14+CD16+单核细胞与其他PBMC人群相比表达更高水平的TNFα (n = 3, p <0.05)和FASL (n = 3, p <0.05)。胆汁酸应激肝细胞通过JNK/c- jun介导的CCL2 (n = 5-20, p <0.0001)和CCL3 (n = 5-20, p <0.05)上调驱动CD14+CD16+单核细胞浸润。结论CD14+CD16+单核细胞浸润促进肝细胞坏死,加重胆汁淤积性肝损伤。因此,靶向它们的趋化性是对抗胆汁淤积的一种有希望的治疗策略。影响和意义本研究揭示了CD14+CD16+单核细胞衍生的肿瘤坏死因子- α (TNFα)和因子相关凋亡配体(FASL)通过受体相互作用丝氨酸/苏氨酸蛋白激酶(RIPK)-1 - ripk3混合谱系激酶结构域样蛋白(MLKL)轴驱动肝细胞坏死凋亡,建立了这些浸润性单核细胞作为胆汁淤积性肝损伤的重要介质。胆汁酸应激肝细胞通过JNK/c-Jun信号上调CCL2和CCL3,促进CD14+CD16+单核细胞浸润。这些发现强调了调节单核细胞浸润或坏死下垂途径以缓解疾病进展的治疗潜力,为治疗OC和相关肝脏病变提供了新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


