Diastereoselective synthesis of 3-substituted-3-hydroxy oxindoles from atropisomeric N-aryl isatin bearing an ortho-dimethylamino group
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
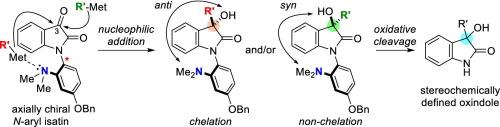
3-Substituted-3-hydroxy oxindoles were synthesized via diastereoselective nucleophilic additions to an axially chiral racemic N-aryl isatin bearing an ortho-dimethylamino group on a p-(benzyloxy)aryl moiety. A switch in diastereoselectivity was observed (up to anti:syn = 29:71 to 74:26) depending on the organometallic reagent (RLi or RMgX). The p-(benzyloxy)aryl moiety was readily removed via a mild two-step sequence to afford N![]() H oxindole.
H oxindole.
含邻二甲胺基的阿托罗二聚体n -芳基isatin非对映选择性合成3-取代-3-羟基氧吲哚
3-取代-3-羟基氧吲哚是通过非对映选择性的亲核加成合成的,在对(苯氧基)芳基上有一个邻二甲氨基。根据有机金属试剂(RLi或RMgX)的不同,观察到非对映选择性的切换(高达anti:syn = 29:71至74:26)。对(苯氧基)芳基部分很容易通过温和的两步序列去除,得到NH氧吲哚。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


