Core-shell engineered Col/Cs@ECM microspheres for macrophage-targeted intracellular drug release in RA therapy
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The imbalance of macrophage polarization between M1 and M2 phenotypes in rheumatoid arthritis (RA) results in a persistent inflammatory cascade. Activating M2 anti-inflammatory polarization, which remove excess extracellular matrix (ECM) via phagocytosis, represents a potential therapeutic target for RA. This study introduces Col/Cs@ECM microspheres, a novel drug delivery system designed for macrophage recognition via a tailored ECM surface, enhancing phagocytic efficiency and accumulation. Moreover, the Col/Cs@ECM microspheres are composed of biocompatible and fully degradable materials, ensuring their safety profile within the physiological environment. Following cell phagocytosis, the collagen/chitosan (Col/Cs) core release the drug (Dexamethasone, Dex) intracellularly to inhibit M1 polarization by inhibiting the NF-κB signaling pathway and to facilitate M2 polarization. This macrophage targeted and intracellular release approach offers a significant advantage over traditional medications by reducing systemic side effects and improving the therapeutic index. The strategy prompts macrophages to express anti-inflammatory cytokines like IL-10 while suppressing pro-inflammatory cytokines such as TNF-α, thereby remodeling the immune microenvironment. Additionally, the specially engineered ECM shell of the microspheres extends the anti-inflammatory response by prolonging macrophage lifespan, a feature that is not present in conventional treatments. This results in improved treatment outcomes in an in vivo RA animal model. This research presents a possible intracellular anti-inflammatory treatment approach for rheumatoid arthritis injection therapy with the potential to outperform existing treatments in terms of efficacy and safety.
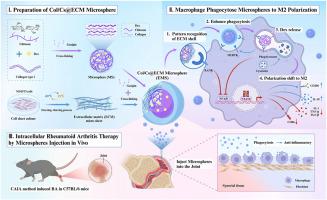
核壳工程Col/Cs@ECM微球用于RA治疗中巨噬细胞靶向细胞内药物释放
类风湿性关节炎(RA)中巨噬细胞M1和M2表型之间的极化不平衡导致持续的炎症级联反应。激活M2抗炎极化,通过吞噬作用去除多余的细胞外基质(ECM),代表了RA的潜在治疗靶点。本研究介绍了Col/Cs@ECM微球,这是一种新型的药物递送系统,旨在通过定制的ECM表面识别巨噬细胞,提高吞噬效率和积累。此外,Col/Cs@ECM微球由生物相容性和完全可降解的材料组成,确保其在生理环境中的安全性。细胞吞噬后,胶原/壳聚糖(Col/Cs)内核在细胞内释放药物(Dexamethasone, Dex),通过抑制NF-κB信号通路抑制M1极化,促进M2极化。与传统药物相比,这种巨噬细胞靶向和细胞内释放方法具有显著的优势,减少了全身副作用,提高了治疗指标。该策略促使巨噬细胞表达IL-10等抗炎细胞因子,抑制TNF-α等促炎细胞因子,从而重塑免疫微环境。此外,微球的特殊设计的ECM外壳通过延长巨噬细胞的寿命来延长抗炎反应,这是传统治疗中不存在的特征。这在体内类风湿性关节炎动物模型中改善了治疗结果。本研究提出了一种可能的细胞内抗炎治疗方法,用于类风湿关节炎注射治疗,在疗效和安全性方面有可能优于现有的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


