Efficient radiocobalt removal from seawater and radioactive liquid waste using a radiation-stable layered metal sulfide ion exchanger
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Radiocobalt (e.g., 58Co and 60Co), a critical neutron-activated corrosion product in the nuclear industry, poses great challenges due to high toxicity. Effective removal of radioactive Co2+ from complex environments is significantly urgent. Metal sulfide ion exchangers (MSIEs) are promising adsorbents; however, limited research has focused on Co2+ removal, especially from seawater and real-world radioactive waste. Herein, a layered K2Cu2Sn2S6 (CTS-1) was reported for the efficient capture of Co2+. The charge-balancing K+ ions are exchangeable, endowing CTS-1 with Co2+ capture ability via an ion exchange mechanism. This mechanism leads to the releasing K+ into solution and concurrently immobilizing Co2+, which was confirmed macroscopically and microscopically. CTS-1 exhibited Langmuir maximum adsorption capacities of 33.96 and 20.72 mg/g in deionized water and seawater at 25 °C, respectively; the overall adsorption kinetics were similar in both matrices. CTS-1 displayed excellent pH durability with >99 % removal of Co2+ at pH 4–8 and high selectivity for Co2+ in various natural matrices. Impressively, in seawater (C0Co = 5 mg/L), CTS-1 achieved 97.38 % of Co2+ removal with a distribution coefficient (Kd) up to 3.817 × 104 mL/g, surpassing many existing adsorbents. Furthermore, CTS-1 exhibited radiation resistance; its structure and adsorption performance remained largely unchanged after a gamma radiation dose of 200 kGy. CTS-1 demonstrated the ability to remove nearly 99 % of radiocobalt from real-world radioactive wastewater, while also showing removal efficiency for other radionuclides like 51Cr and 58Mn. Overall, this work provides a highly promising material to tackle radiocobalt contamination in radioactive waste, greatly promoting environmental protection and safety for the radioactive industry.
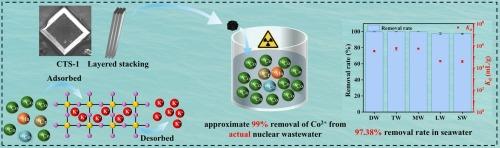
使用辐射稳定的层状金属硫化物离子交换器从海水和放射性废液中有效去除放射性钴
放射性钴(如58Co和60Co)是核工业中重要的中子活化腐蚀产物,由于其高毒性,给核工业带来了巨大的挑战。从复杂的环境中有效地去除放射性Co2+是非常紧迫的。金属硫化物离子交换剂是一种很有前途的吸附剂;然而,有限的研究集中在二氧化碳+的去除,特别是从海水和现实世界的放射性废物。本文报道了一种层状的K2Cu2Sn2S6 (CTS-1),用于有效捕获Co2+。电荷平衡的K+离子是可交换的,通过离子交换机制赋予CTS-1捕获Co2+的能力。这一机制导致K+释放到溶液中,同时固定化Co2+,宏观和微观上证实了这一机制。CTS-1在25℃去离子水和海水中的Langmuir最大吸附量分别为33.96和20.72 mg/g;两种基质的总体吸附动力学相似。CTS-1表现出优异的pH耐久性,在pH 4-8下Co2+去除率达99%,在各种天然基质中对Co2+有很高的选择性。令人印象深刻的是,在海水(C0Co = 5 mg/L)中,CTS-1的Co2+去除率达到97.38%,分配系数(Kd)高达3.817 × 104 mL/g,超过了许多现有的吸附剂。CTS-1具有抗辐射能力;辐照剂量为200kgy后,其结构和吸附性能基本保持不变。CTS-1展示了从实际放射性废水中去除近99%放射性钴的能力,同时也显示了对51Cr和58Mn等其他放射性核素的去除效率。总之,本研究为解决放射性废物中的放射性钴污染提供了一种极具前景的材料,极大地促进了放射性工业的环境保护和安全。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


