The cleavage of dsRNA by StaufenC into siRNA affects the RNAi efficiency in the 28-spotted ladybeetle, Henosepilachna vigintioctopunctata
IF 4
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
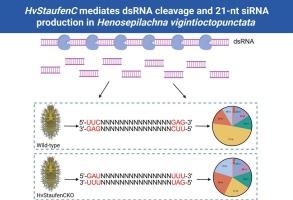
The efficacy of RNA interference (RNAi) in insects hinges critically on the precise conversion of double-stranded RNA (dsRNA) into small interfering RNAs (siRNAs), which are essential for initiating effective gene silencing. While our recent work identified HvStaufenC as a key regulator of RNAi efficiency in the coleopteran pest Henosepilachna vigintioctopunctata, the underlying mechanism remained unknown. To define HvStaufenC's role, we employed complementary in vitro and in vivo strategies. In vitro, recombinant HvStaufenC protein efficiently processed dsRNA into siRNAs primarily under 35 nucleotides (nt) in length. Crucially, this catalytic activity was completely abolished in a mutant protein harboring disruptions in its essential LNN motif (HvStaufenC-Mutant). In vivo, small RNA sequencing of larvae injected with dsRNA targeting HvTH gene revealed striking differences: wild-type produced siRNAs overwhelmingly enriched for 21-nt species (49.08 % of 19–25-nt siRNAs), characterized by 5’-GAU/UUG/GAA and 3’-UUU/GUU/UUG cleavage motifs. In contrast, HvStaufenC knockout mutants (HvStaufenCKO) showed significantly impaired 21-nt siRNA generation (22.73 %) and exhibited altered cleavage preferences (5’-UUC/GAU/AUU; 3′-GAG/UAC/GGA). Functional validation confirmed the biological significance of these processing differences. Chimeric dsRNA constructs incorporating most abundant wild-type-derived 21-nt siRNA sequences triggered significantly stronger RNAi effects compared to constructs based on sequences derived from HvStaufenCKO mutants. Moreover, no phenotypic changes were observed in HvStaufenCKO 3rd instar larvae following the injection of chimeric dsRNAs. Collectively, these findings demonstrate that HvStaufenC is indispensable for efficient RNAi in H. vigintioctopunctata. It specifically mediates dsRNA cleavage to generate functional 21-nt siRNAs with distinct sequence motifs, providing novel mechanistic insights into RNAi regulation within insects.
StaufenC将dsRNA裂解为siRNA影响了28斑瓢虫(Henosepilachna vigintioctopunctata)的RNAi效率
RNA干扰(RNAi)在昆虫中的作用关键取决于双链RNA (dsRNA)精确转化为小干扰RNA (sirna),这是启动有效基因沉默所必需的。虽然我们最近的研究发现HvStaufenC在鞘翅目害虫Henosepilachna viintioctopunctata中是RNAi效率的关键调节因子,但其潜在机制尚不清楚。为了确定HvStaufenC的作用,我们采用了互补的体外和体内策略。在体外,重组HvStaufenC蛋白有效地将dsRNA加工成长度小于35个核苷酸(nt)的sirna。至关重要的是,这种催化活性在其基本LNN基序被破坏的突变蛋白(hvstaufenca - mutant)中被完全消除。在体内,注射了靶向HvTH基因的dsRNA的幼虫的小RNA测序显示出惊人的差异:野生型产生的sirna绝大多数富集于21-nt物种(占19-25-nt sirna的49.08%),其特征是5 ' -GAU/UUG/GAA和3 ' -UUU/GUU/UUG切割基序。相比之下,HvStaufenCKO敲除突变体(HvStaufenCKO)显示21-nt siRNA生成显著受损(22.73%),并表现出改变的切割偏好(5 ' -UUC/GAU/AUU; 3 ' -GAG/UAC/GGA)。功能验证证实了这些加工差异的生物学意义。与基于HvStaufenCKO突变体序列的嵌合dsRNA构建物相比,包含最丰富的野生型衍生的21-nt siRNA序列的嵌合dsRNA构建物触发的RNAi效应明显更强。此外,注射嵌合dsRNAs后,HvStaufenCKO 3龄幼虫未观察到表型变化。总的来说,这些发现表明HvStaufenC对于H. vigintioctopunctata的高效RNAi是不可或缺的。它特异性地介导dsRNA裂解,产生具有不同序列基序的功能性21-nt sirna,为昆虫体内RNAi调控提供了新的机制见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


