Optimized electrochemical performance of Pr2NiO4 via partial Ni-substitution with molybdenum
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
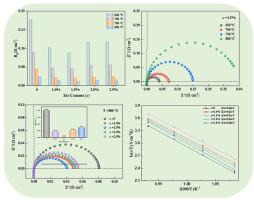
This research aimed to develop novel Pr2NiO4-based cathodes for solid oxide fuel cells (SOFCs) through partial substitution of Ni with Mo. Pr2Ni1-xMoxO4 (x = 0, 0.01, 0.015, 0.02, 0.025) cathode powders were prepared via the sol-gel combustion method. The phase structure of the as-prepared powders was characterized by X-ray diffraction (XRD), and the microstructure was examined using scanning electron microscopy (SEM). Electrical conductivity was measured by the DC four-terminal method, and the electrochemical performance of the half-cells was evaluated using electrochemical impedance spectroscopy (EIS). The Pr2Ni1-xMoxO4 powders exhibiting good chemical stability and thermal expansion matching with the Ce0.8Gd0.2O1.9electrolyte. Mo doping significantly improved the electrical conductivity of Pr2NiO4. The electrical conductivity of the Pr2Ni0·985Mo0·015O4 (x = 0.015) reached 129.07 S cm−1 at 450 °C. EIS results revealed that Mo doping markedly reduced the polarization resistance (Rp), and the lowest value obtained was 0.021 Ω cm2 for the Pr2Ni0·985Mo0·015O4 sample. These findings suggest that Mo-doped Pr2NiO4 cathodes possess enhanced oxygen reduction reaction activity.
钼部分取代镍优化了Pr2NiO4的电化学性能
采用溶胶-凝胶燃烧法制备了Pr2Ni1-xMoxO4 (x = 0、0.01、0.015、0.02、0.025)阴极粉末,制备了新型固体氧化物燃料电池(SOFCs)用Pr2Ni1-xMoxO4阴极粉末。用x射线衍射(XRD)表征了所制备粉体的相结构,并用扫描电子显微镜(SEM)对其微观结构进行了表征。采用直流四端法测定了半电池的电导率,采用电化学阻抗谱(EIS)评价了半电池的电化学性能。制备的Pr2Ni1-xMoxO4粉末具有良好的化学稳定性和热膨胀性能,与ce0.8 gd0.2 2o1.9电解质相匹配。Mo掺杂显著提高了Pr2NiO4的电导率。在450℃时,Pr2Ni0·985Mo0·015O4 (x = 0.015)的电导率达到129.07 S cm−1。EIS结果表明,Mo的掺杂显著降低了Pr2Ni0·985Mo0·015O4样品的极化电阻(Rp),其最小值为0.021 Ω cm2。这些结果表明,掺杂mo的Pr2NiO4阴极具有增强的氧还原反应活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


