Anoectochilus roxburghii polysaccharide alleviates DSS-induced ulcerative colitis by modulating the MAPK/NF-κB signaling pathway and gut microbiota
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Ulcerative colitis (UC) involves mucosal barrier disruption, immune dysregulation, and gut dysbiosis, with current therapies limited by cost and side effects. This study evaluated the effects of Anoectochilus roxburghii polysaccharide (ARP)—a glucan-rich heteropolysaccharide (888 Da–2.43 × 106 Da)—on dextran sulfate sodium (DSS)-induced murine colitis model. ARP (150–300 mg/kg) markedly alleviated colitis by reducing the disease activity index, restoring colon length, and improving histopathology. ARP enhanced intestinal barrier integrity by upregulating ZO-1, OCLN, and MUC2, while suppressing pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), M1 macrophage polarization, and MAPK/NF-κB signaling. Transcriptomic and immunoblot analyses confirmed the downregulation of key inflammatory mediators, including phosphorylated p38, JNK, and IκBα. ARP also modulated gut microbiota, increasing Bacteroidota and decreasing Proteobacteria abundance. These results provide integrated evidence that ARP exerts multimodal protective effects in UC by targeting barrier dysfunction, immune hyperactivation, and microbial dysbiosis, establishing a mechanistic basis for developing ARP as a functional food and natural dietary intervention for UC.
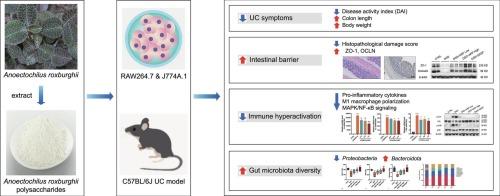
刺梨多糖通过调节MAPK/NF-κB信号通路和肠道菌群减轻dss诱导的溃疡性结肠炎
溃疡性结肠炎(UC)涉及粘膜屏障破坏、免疫失调和肠道生态失调,目前的治疗方法受成本和副作用的限制。本研究评价了富葡聚糖杂多糖(888 Da - 2.43 × 106 Da)山梨多糖(ARP)对葡聚糖硫酸钠(DSS)诱导的小鼠结肠炎模型的影响。ARP (150 ~ 300 mg/kg)通过降低疾病活动性指数、恢复结肠长度、改善组织病理学来显著缓解结肠炎。ARP通过上调ZO-1、OCLN和MUC2增强肠道屏障完整性,同时抑制促炎细胞因子(IL-1β、IL-6和TNF-α)、M1巨噬细胞极化和MAPK/NF-κB信号传导。转录组学和免疫印迹分析证实了关键炎症介质的下调,包括磷酸化的p38、JNK和i - κ b α。ARP还能调节肠道菌群,增加拟杆菌群,减少变形菌群。这些结果提供了综合证据,表明ARP在UC中通过针对屏障功能障碍、免疫过度激活和微生物生态失调发挥多模式的保护作用,为开发ARP作为UC的功能性食品和天然饮食干预奠定了机制基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


