Nanogram-level green spectrofluorimetric method for simultaneous quantification of tadalafil and silodosin in pharmaceuticals, environmental matrices and content uniformity testing
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
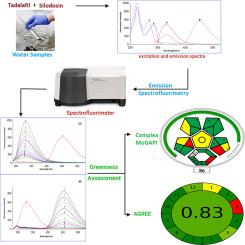
A novel, eco-friendly spectrofluorimetric method has been developed and validated for the first time for simultaneous determination of tadalafil (TDF) and silodosin (SLD) in pharmaceutical dosage forms and environmental water samples without the need for prior separation. This innovative approach exploits the emission fluorescence of both drugs, employing excitation at 270 nm and emission detection at 328 nm for TDF and 454 nm for SLD. The method demonstrated excellent linearity across the concentration ranges of 50.0–1200.0 ng mL-1 for TDF and 1.0–300.0 ng mL-1 for SLD (r > 0.9999), with remarkable limits of detection of 10.94 ng mL-1 and 0.28 ng mL-1, respectively. The high sensitivity and precision (RSD < 0.70 %), with recovery rates ranging from 99.50 % to 101.00 % in pharmaceuticals and 97.31 % to 102.83 % in environmental water emphasize the method’s robustness and practical applicability for therapeutic drug monitoring and environmental surveillance. Application to content uniformity testing of tablet formulations, in accordance with British Pharmacopoeia guidelines, further demonstrates the method’s versatility. Greenness assessments using the AGREE and complex moGAPI metrics, alongside operational efficiency evaluation through the Click Analytical Chemistry Index (CACI), confirm the method’s sustainability and minimal environmental impact. Fully compliant with ICH Q2(R1) guidelines, this validated protocol offers a pioneering, rapid, and sustainable alternative for routine quality control and environmental monitoring.
纳克级绿色荧光光谱法同时定量药品、环境基质中他达拉非和西洛多辛含量均匀性试验
本文首次建立了一种新型、环保的荧光光谱法,可同时测定药物剂型和环境水样中的他达拉非(TDF)和西洛多辛(SLD),而无需事先分离。这种创新的方法利用了两种药物的发射荧光,采用270 nm的激发和328 nm的发射检测,TDF和454 nm的SLD。该方法在TDF浓度范围为50.0 ~ 1200.0 ng mL-1和SLD浓度范围为1.0 ~ 300.0 ng mL-1 (r > 0.9999)具有良好的线性关系,检测限分别为10.94 ng mL-1和0.28 ng mL-1。该方法具有较高的灵敏度和精密度(RSD < 0.70%),在药品和环境水中的回收率分别为99.50% ~ 101.00%和97.31% ~ 102.83%,具有较好的鲁棒性和实用性。应用于片剂配方的含量均匀性测试,根据英国药典指南,进一步证明了该方法的通用性。使用AGREE和复杂的moGAPI指标进行的绿色评估,以及通过点击分析化学指数(CACI)进行的操作效率评估,证实了该方法的可持续性和最小的环境影响。该方案完全符合ICH Q2(R1)指南,为常规质量控制和环境监测提供了一种开创性、快速和可持续的替代方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


