Learning ecosystem-scale dynamics from microbiome data with MDSINE2
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
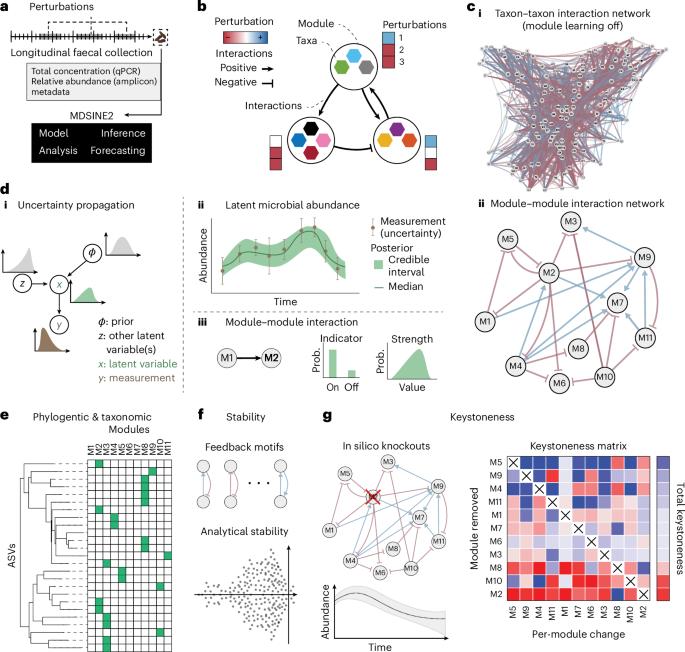
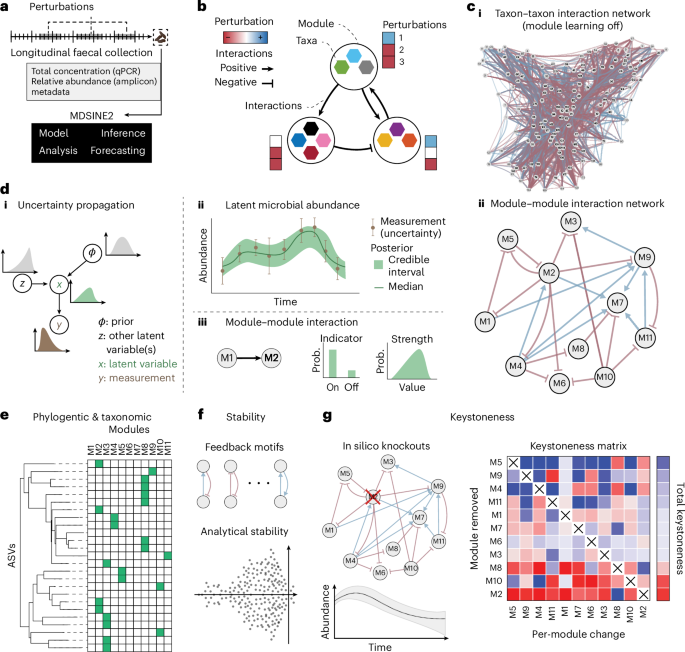
Although dynamical systems models are a powerful tool for analysing microbial ecosystems, challenges in learning these models from complex microbiome datasets and interpreting their outputs limit use. We introduce the Microbial Dynamical Systems Inference Engine 2 (MDSINE2), a Bayesian method that learns compact and interpretable ecosystems-scale dynamical systems models from microbiome timeseries data. Microbial dynamics are modelled as stochastic processes driven by interaction modules, or groups of microbes with similar interaction structure and responses to perturbations, and additionally, noise characteristics of data are modelled. Our open-source software package provides multiple tools for interpreting learned models, including phylogeny/taxonomy of modules, and stability, interaction topology and keystoneness. To benchmark MDSINE2, we generated microbiome timeseries data from two murine cohorts that received faecal transplants from human donors and were then subjected to dietary and antibiotic perturbations. MDSINE2 outperforms state-of-the-art methods and identifies interaction modules that provide insights into ecosystems-scale interactions in the gut microbiome. This Bayesian statistical method uses timeseries microbiome data to infer interaction modules and is tested using a faecal transplant experiment in mice.
从微生物组数据学习生态系统尺度动态与MDSINE2
尽管动力系统模型是分析微生物生态系统的强大工具,但从复杂的微生物组数据集中学习这些模型并解释其输出的挑战限制了其使用。我们介绍了微生物动力系统推理引擎2 (MDSINE2),这是一种贝叶斯方法,可以从微生物组时间序列数据中学习紧凑且可解释的生态系统尺度动力系统模型。微生物动力学建模为由相互作用模块或具有相似相互作用结构和对扰动响应的微生物群驱动的随机过程,此外,还建模了数据的噪声特征。我们的开源软件包提供了多种解释学习模型的工具,包括模块的系统发育/分类、稳定性、交互拓扑和关键点性。为了对MDSINE2进行基准测试,我们从两个接受人类供体粪便移植的小鼠队列中生成了微生物组时间序列数据,然后对饮食和抗生素进行了扰动。MDSINE2优于最先进的方法,并确定相互作用模块,提供对肠道微生物群中生态系统规模相互作用的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


