A pathological role of O-GlcNAcylation-driven TR11B production and function in lung adenocarcinoma
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
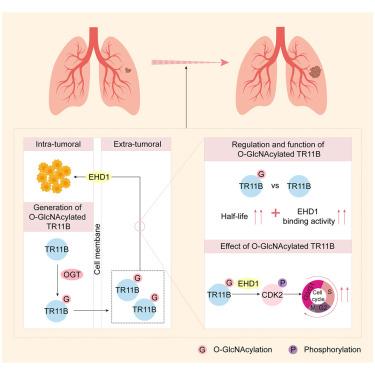
Cytokines link inflammation to tumorigenesis, but the role of post-translational modifications in regulating their function within the extra-tumoral environment remains poorly defined. Here, we identify tumor-derived tumor necrosis factor (TNF) receptor superfamily member 11B (TR11B) as a key driver of lung adenocarcinoma (LUAD) progression and therapeutic resistance. Mechanistically, O-GlcNAc transferase (OGT)-mediated O-GlcNAcylation at serine 151 stabilizes TR11B and facilitates its interaction with the membrane protein EPS15 homology domain-containing protein 1 (EHD1), promoting cyclin dependent kinase 2 (CDK2) phosphorylation and cell cycle progression. Clinically, elevated O-GlcNAcylated TR11B correlates with advanced LUAD. Genetic deletion of Ogt suppresses tumor development in LUAD mouse models. Importantly, celecoxib, an U.S. Food and Drug Administration (FDA)-approved drug, inhibits O-GlcNAcylation and exerts antitumor effects. These findings reveal a pathological role for cytokine O-GlcNAcylation in LUAD and identify this axis as a potential therapeutic target.
o - glcn酰化驱动TR11B产生和功能在肺腺癌中的病理作用
细胞因子将炎症与肿瘤发生联系起来,但在肿瘤外环境中,翻译后修饰在调节其功能中的作用仍不清楚。在这里,我们发现肿瘤源性肿瘤坏死因子(TNF)受体超家族成员11B (TR11B)是肺腺癌(LUAD)进展和治疗耐药的关键驱动因素。从机制上说,O-GlcNAc转移酶(OGT)介导的丝氨酸151处的o - glcn酰化稳定了TR11B,促进了其与膜蛋白EPS15同源结构域蛋白1 (EHD1)的相互作用,促进了周期蛋白依赖性激酶2 (CDK2)的磷酸化和细胞周期的进展。临床上,o - glcn酰化TR11B升高与晚期LUAD相关。在LUAD小鼠模型中,Ogt基因缺失抑制肿瘤的发展。重要的是,塞来昔布是美国食品和药物管理局(FDA)批准的药物,可抑制o - glcn酰化并发挥抗肿瘤作用。这些发现揭示了细胞因子o - glcn酰化在LUAD中的病理作用,并将该轴确定为潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


