The synthesis and evaluation of new N-(pyrazin-2-yl)-4-aminopyrimidine derivatives targeted EGFR and JAK
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
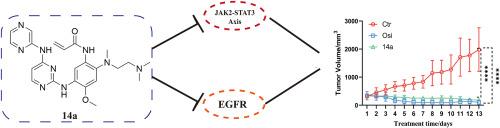
To discover novel active compounds against non-small cell lung cancer, thirty one amino pyrazine derivatives were synthesized and evaluated as inhibitors against EGFR-mutation cancers. The optimal compound 14a, exhibited 15.4 nM and 18.5 nM of the IC50 values against PC9 and H1975 cancer cell lines, respectively. In H1975 xenograft nude mice, 14a exhibited 90.0 % of the TGI when oral administration at dosage of 10 mg/kg. Kinase activity assay showed that 14a not only has excellent inhibitory activity against EGFR kinase, but also has good activity against JAK2 and JAK3 kinases, exhibiting a dual target characteristics. Mechanism study indicated that 14a could inhibit the phosphorylation of EGFR protein and decrease the active form p-JAK2 for JAK2, induce an increase in intracellular ROS, which may disrupt the balance of the redox system in cancer cells and cause the death of tumor cells. In addition, 14a could increase cellular lipid oxide MDA, meanwhile decrease GSH content, which suggests that 14a have caused ferroptosis in cancer cells, Finally, 14a exhibited high selectivity towards EGFRwt cells with a selectivity ratio of 583.76, which is significant to avoid toxic side effects of drugs.
靶向EGFR和JAK的新型N-(吡嗪-2-酰基)-4-氨基嘧啶衍生物的合成与评价
为了发现抗非小细胞肺癌的新活性化合物,合成了31种氨基吡嗪衍生物,并对其作为egfr突变肿瘤的抑制剂进行了评价。最佳化合物14a对PC9和H1975癌细胞的IC50值分别为15.4 nM和18.5 nM。在H1975异种移植裸鼠中,14a口服剂量为10 mg/kg时,TGI为90.0%。激酶活性测定表明,14a不仅对EGFR激酶具有良好的抑制活性,而且对JAK2和JAK3激酶也具有良好的抑制活性,表现出双靶标特性。机制研究表明,14a可抑制EGFR蛋白磷酸化,降低JAK2的活性形式p-JAK2,诱导细胞内ROS增加,从而破坏癌细胞氧化还原系统的平衡,导致肿瘤细胞死亡。此外,14a能增加细胞脂质氧化物MDA,同时降低GSH含量,提示14a引起癌细胞铁下垂。最后,14a对EGFRwt细胞具有很高的选择性,选择性比为583.76,这对于避免药物毒副作用具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


