Lipid nanoparticle mediated delivery of dsDNA to the murine retina
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Gene therapy has become a successful tool for treating inherited retinal diseases (IRDs). To date, recombinant adeno-associated virus (rAAV)- mediated delivery is the preferred method for gene transfer; however, its limited payload capacity restricts its use for treatment to causative loci under 5 kb. Recent advances in gene therapy tools have demonstrated the success of non-viral delivery vectors with lipid-based nanoparticles (LNPs) at the forefront. Owing to the SARS-CoV-2 vaccine, LNPs have already demonstrated clinical safety, however in regard to IRDs, the LNPs are limited to RPE and Muller glia cells, and their application has been limited to transient RNA delivery. Previously, we have reported that the introduction of N-Hydroxysuccinamide (NHS) functionalized PEG lipid (DSPE-PEG2K-NHS) to the LNPs (called LNPx) improved their overall transfection rate and widened their transfection efficiency to include not only RPE but photoreceptor cells. Taken together, this could expand LNP utility and allow for the treatment of many prevalent IRDs. Herein, we utilized LNPx to demonstrate the feasibility and safety of dsDNA delivery after subretinal injection into the murine retina, through a combination of multimodal in vivo imaging and post-mortem histology.

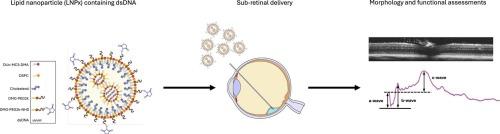
脂质纳米颗粒介导dsDNA向小鼠视网膜的传递
基因治疗已成为治疗遗传性视网膜疾病的成功手段。迄今为止,重组腺相关病毒(rAAV)介导的递送是基因转移的首选方法;然而,其有限的有效载荷能力限制了其用于治疗5 kb以下致病位点的使用。基因治疗工具的最新进展表明,以脂质纳米颗粒(LNPs)为基础的非病毒传递载体取得了成功。由于SARS-CoV-2疫苗,LNPs已经证明了临床安全性,但就IRDs而言,LNPs仅限于RPE和Muller胶质细胞,其应用仅限于瞬时RNA递送。在此之前,我们报道了将n -羟基琥珀酰胺(NHS)功能化的PEG脂质(DSPE-PEG2K-NHS)引入LNPs(称为LNPx),提高了LNPs (LNPx)的整体转染率,并扩大了其转染效率,不仅包括RPE,还包括光感受器细胞。综上所述,这可以扩大LNP的效用,并允许治疗许多常见的ird。在此,我们利用LNPx通过多模态体内成像和死后组织学相结合,证明了将dsDNA注射到小鼠视网膜下后传递到小鼠视网膜的可行性和安全性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


