The hidden impact of intrauterine growth restriction in the pathogenesis of metabolic syndrome: Functional and structural alterations in rat visceral adipose tissue
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Individuals born after intrauterine growth restriction (IUGR) have a higher risk of developing metabolic syndrome (MetS) in adulthood. In a rat model, male IUGR offspring exhibit MetS features—including elevated systolic blood pressure, glucose intolerance, non-alcoholic fatty liver disease, and increased visceral adipose tissue (VAT)—by 6 months of age. Female offspring, however, do not. While higher VAT is associated with MetS, its role in IUGR-induced metabolic disorders remains unclear. The objective is to examine structural changes and mechanisms in VAT associated with metabolic disorders in IUGR rats. IUGR was induced via a maternal low-protein (9% casein) and compared to a control diet (23% casein). VAT was collected from 6-month-old offspring. Adipocyte hyperplasia and hypertrophy were analyzed using Ki-67 and hematoxylin/eosin (H/E) staining. Adipogenesis (PPAR-γ by Western blot; ZFP423 by RT-qPCR), inflammation (IL-6, TNF-α by RT-qPCR), macrophage markers (CD68, ITGAM, ITGAX by RT-qPCR), oxidative stress (superoxide anion via hydroethidine; Cu/Zn SOD, catalase by Western blot), and senescence (lipofuscin via autofluorescence, crown-like structures by H/E, p16INK4a, p21WAF1, Sirtuin-1 by Western blot) were evaluated. IUGR males showed increased adipocyte proliferation, hypertrophy, and upregulated adipogenic markers (PPAR-γ, ZFP423; P<.05, P<.001). Inflammatory and macrophage markers were elevated (P<.05), along with superoxide anion production, Cu/Zn SOD and catalase protein expression (P<.05). Senescence indicators—including lipofuscin, crown-like structures, p16INK4a, p21WAF1, and Sirtuin-1 were also upregulated (all P<.05). No significant changes were observed in females. VAT from male IUGR offspring exhibits increased adipogenesis, inflammation, oxidative stress, and premature senescence, suggesting a mechanistic link to their higher MetS susceptibility.
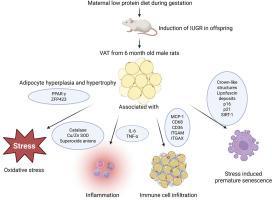
宫内生长受限对代谢综合征发病机制的潜在影响:大鼠内脏脂肪组织的功能和结构改变
背景:宫内生长受限(IUGR)后出生的个体在成年期发生代谢综合征(MetS)的风险较高。在大鼠模型中,IUGR雄性后代在6个月大时表现出MetS特征,包括收缩压升高、葡萄糖耐受不良、非酒精性脂肪性肝病和内脏脂肪组织(VAT)增加。然而,雌性后代却没有。虽然较高的VAT与MetS相关,但其在iugr诱导的代谢紊乱中的作用尚不清楚。目的:探讨IUGR大鼠代谢紊乱与VAT的结构变化及其机制。方法:通过母体低蛋白(9%酪蛋白)诱导IUGR,并与对照饮食(23%酪蛋白)进行比较。增值税从6个月大的幼仔身上收取。Ki-67和苏木精/伊红(H/E)染色分析脂肪细胞增生和肥厚。评估脂肪生成(Western blot检测PPAR-γ, RT-qPCR检测ZFP423)、炎症(RT-qPCR检测IL-6、TNF-α)、巨噬细胞标志物(RT-qPCR检测CD68、ITGAM、ITGAX)、氧化应激(氢乙胺检测超氧阴离子,Western blot检测Cu/Zn SOD、过氧化氢酶)和衰老(自身荧光检测脂褐素,H/E检测冠状结构,Western blot检测p16INK4a、p21WAF1、Sirtuin-1)。结果:IUGR男性显示脂肪细胞增殖增加,肥大,脂肪生成标志物上调(PPAR-γ, ZFP423; p < 0.05, p < 0.001)。炎症和巨噬细胞标志物升高(p < 0.05),超氧阴离子生成、Cu/Zn SOD和过氧化氢酶蛋白表达升高(p < 0.05)。脂褐素、冠状结构、p16INK4a、p21WAF1、Sirtuin-1等衰老指标也上调(p < 0.05)。在女性中没有观察到明显的变化。结论:IUGR雄性后代的VAT表现出脂肪生成、炎症、氧化应激和过早衰老的增加,这表明它们与更高的MetS易感性有机制联系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


