HIF-1α in glioblastoma multiforme cells: Mechanisms involving the Wnt/β-catenin signaling pathway
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Glioblastoma multiforme (GBM) is a rapidly progressing brain malignancy, with its progression closely tied to a hypoxic microenvironment. Hypoxia-inducible factor-1α (HIF-1α) acts as a vital regulator in tumor adaptation to low oxygen levels, and its relationship with the Wnt/β-catenin signaling pathway exerts significant functions in the malignant properties of GBM. In this research, Western blot and qRT-PCR were applied to check β-catenin and HIF-1α expression in GBM. How HIF-1α influenced GBM cell behavior was investigated in vitro through the construction of U251 cell models with HIF-1α overexpression and knockdown, accompanied by assessments of cell proliferation, migration, invasion, and apoptosis. Additionally, co-immunoprecipitation (Co-IP) experiments were leveraged for checking the interaction of β-catenin and HIF-1α. This study demonstrated that HIF-1α and β-catenin were markedly upregulated in GBM tissues relative to normal controls, and their expression was positively correlated at the transcriptional level. In oxygen-limited environments, HIF-1α expression was significantly enhanced, and the Wnt signaling pathway was activated through stabilizing β-catenin. Functional experiments showed that HIF-1α overexpression facilitated cell proliferation, migration, and invasion, while inhibiting apoptosis; conversely, HIF-1α knockdown led to a significant reduction in these processes. Taken together, HIF-1α regulates the Wnt/β-catenin signaling pathway via its engagement with β-catenin, thereby promoting GBM proliferation and invasion, and inhibiting apoptosis. These findings emphasize the importance of HIF-1α in GBM advancement and suggest novel therapeutic strategies targeting HIF-1α and the Wnt/β-catenin pathway.
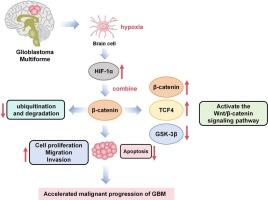
胶质母细胞瘤多形性细胞中的HIF-1α:涉及Wnt/β-catenin信号通路的机制。
多形性胶质母细胞瘤(GBM)是一种进展迅速的脑恶性肿瘤,其进展与缺氧微环境密切相关。缺氧诱导因子-1α (HIF-1α)是肿瘤适应低氧水平的重要调节因子,其与Wnt/β-catenin信号通路的关系在GBM的恶性特性中发挥重要作用。本研究采用Western blot和qRT-PCR检测β-catenin和HIF-1α在GBM中的表达。通过构建HIF-1α过表达和低表达的U251细胞模型,在体外研究HIF-1α对GBM细胞行为的影响,并评估细胞增殖、迁移、侵袭和凋亡。此外,利用共免疫沉淀(Co-IP)实验检测β-catenin与HIF-1α的相互作用。本研究表明,与正常对照相比,HIF-1α和β-catenin在GBM组织中的表达明显上调,且在转录水平上呈正相关。缺氧环境下,HIF-1α表达显著增强,通过稳定β-catenin激活Wnt信号通路。功能实验表明,HIF-1α过表达促进细胞增殖、迁移和侵袭,抑制细胞凋亡;相反,HIF-1α敲低导致这些过程显著减少。综上所述,HIF-1α通过与β-catenin的结合调控Wnt/β-catenin信号通路,从而促进GBM的增殖和侵袭,抑制细胞凋亡。这些发现强调了HIF-1α在GBM进展中的重要性,并提出了针对HIF-1α和Wnt/β-catenin通路的新治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


