Reshaping tumor immune microenvironment through ROS-responsive prodrug polyplexes via synergistic effect of CRISPRi system and epigenetic inhibitor for breast cancer therapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Engagement of programmed death-ligand 1 (PD-L1) on tumor cells with its receptor PD-1 on immune cells can transmit an inhibitory signal to induce immune evasion. Although the immune checkpoint inhibitor PD-L1 antibody has shown antitumor capability in clinical treatment, its wide clinical application still faces several side effects and individual selectivity. In our research, we utilized the Clustered Regularly Interspaced Short Palindromic Repeats interference (CRISPRi) system to suppress PD-L1 expression on breast cancer cells (4T1) and combined it with epigenetic inhibitor azacytidine (AZA) for enhanced cancer immunotherapy. Reactive oxygen species (ROS)-responsive poly(β-amino ester) (PBAE)-S-AZA cationic polymeric prodrug was fabricated, which could complex with CRISPRi plasmids to form the composite polyplexes via electrostatic interaction. The composite polyplexes could be taken up by tumor cells with high efficiency, followed by plasmid release with the cooperation of PBAE. The CRISPRi plasmids could lead to PD-L1 downregulation in tumor cells, leading to obvious relief of immune checkpoint blockade. In the meantime, the epigenetic inhibitor AZA was also released from the polyplexes due to the high intracellular ROS level, thereby enhancing the efficacy of immunotherapy via elevating MHC class I expression, enhancing antigen presentation, and inducing dendritic cell (DC) maturation. The ROS-responsive polyplexes helped to realize the combination of genome editing, immunotherapy, and epigenetic regulation. It will provide an effective platform for promoting antitumor treatment and precision medicine.
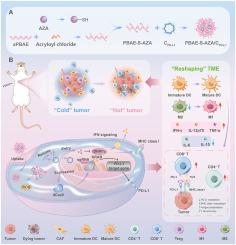
CRISPRi系统与表观遗传抑制剂协同作用,通过ros应答前药复合物重塑肿瘤免疫微环境用于乳腺癌治疗
肿瘤细胞上的程序性死亡配体1 (programmed death-ligand 1, PD-L1)与其免疫细胞上的受体PD-1结合可传递抑制信号,诱导免疫逃逸。尽管免疫检查点抑制剂PD-L1抗体在临床治疗中显示出抗肿瘤能力,但其广泛的临床应用仍面临着一些副作用和个体选择性。在我们的研究中,我们利用聚集规则间隔短回文重复序列干扰(CRISPRi)系统抑制乳腺癌细胞(4T1)上PD-L1的表达,并将其与表观遗传抑制剂阿扎胞苷(AZA)联合用于增强癌症免疫治疗。制备了活性氧(ROS)响应型聚β-氨基酯(PBAE)-S-AZA阳离子高分子前药,可与CRISPRi质粒通过静电相互作用形成复合复合物。复合复合物能被肿瘤细胞高效吸收,并在PBAE的配合下释放质粒。CRISPRi质粒可导致肿瘤细胞中PD-L1的下调,导致免疫检查点阻断的明显缓解。同时,由于细胞内ROS水平高,表观遗传抑制剂AZA也从多丛中释放出来,从而通过提高MHC I类表达、增强抗原呈递和诱导树突状细胞(DC)成熟来增强免疫治疗的效果。ros应答多丛有助于实现基因组编辑、免疫治疗和表观遗传调控的结合。它将为促进抗肿瘤治疗和精准医疗提供一个有效的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


