Bioinspired nanoparticles prevent blue-light-induced skin hyperpigmentation via FZD2-TYR-melanin pathway
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Melanin is the natural physiological defense for skin, but excessive melanin production can result in conditions like hyperpigmentation and melasma. In addition to UV light, visible light, especially blue light, has been recognized as an extra factor contributing to cutaneous hyperpigmentation and premature photoaging. However, there are currently no effective protective agents against blue light. Within this research, we obtained polydopamine nanoparticles (PDA NPs) and cuttlefish ink nanoparticles (CINPs), which are inspired by endogenous melanin. Our research confirmed that PDA NPs and CINPs shared similar structural and functional properties with natural melanin. Otherwise, these nanoparticles not only exhibited excellent photostability and broad-spectrum ultraviolet–visible light absorption capabilities but also possessed superb biocompatibility and antioxidant properties. Experiments conducted in vitro and in vivo verified that PDA NPs and CINPs could effectively hold back melanin production and alleviate pigmentation. Furthermore, we found that the underlying mechanism by which PDA NPs and CINPs reduced melanin formation was through inhibition of the FZD2-TYR-melanin signaling pathway. Taken together, our findings not only demonstrate that PDA NPs and CINPs are powerful anti-blue light agents but also provide an in-depth mechanism of these nanoparticles in the inhibition of pigment formation.
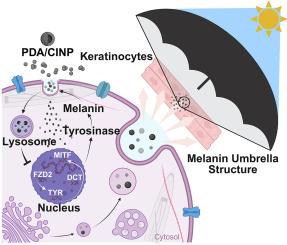
生物激发纳米颗粒通过fzd2 - tyr1 -黑色素途径预防蓝光诱导的皮肤色素沉着
黑色素是皮肤的天然生理屏障,但过多的黑色素产生会导致色素沉着和黄褐斑等疾病。除了紫外线,可见光,尤其是蓝光,也被认为是导致皮肤色素沉着和过早光老化的一个额外因素。然而,目前还没有有效的蓝光防护剂。在本研究中,我们获得了受内源性黑色素启发的聚多巴胺纳米粒子(PDA NPs)和墨鱼墨水纳米粒子(CINPs)。我们的研究证实,PDA NPs和CINPs与天然黑色素具有相似的结构和功能特性。此外,这些纳米颗粒不仅具有优异的光稳定性和广谱紫外可见光吸收能力,而且具有良好的生物相容性和抗氧化性能。体外和体内实验证实,PDA NPs和CINPs能有效抑制黑色素生成,缓解色素沉着。此外,我们发现PDA NPs和CINPs减少黑色素形成的潜在机制是通过抑制fzd2 - tyr -黑色素信号通路。综上所述,我们的研究结果不仅证明了PDA NPs和CINPs是强大的抗蓝光剂,而且还提供了这些纳米颗粒抑制色素形成的深入机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


