Boosting bifunctional and overall water splitting activity by direct fabricating bimetallic metal hydroxide catalyst on nickel foam
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Bimetallic cobalt-nickel metal nitrate hydroxide electrocatalyst fabricated on a conducting nickel foam (NF) displayed excellent bifunctional electrocatalytic activity and improved overall water splitting. X-ray photoelectron spectroscopy (XPS) analysis confirmed the presence of Co and Ni and HR-SEM suggested the formation of crystalline needle nanostructures. The individual CoNH and NiNH required overpotential of 330 and 317 mV in OER and 267 and 293 mV in HER, respectively to produce 50 mA/cm2 current density. In contrast, bimetallic CoNiNH required lower overpotential (292 mV in OER and 242 mV in HER) to achieve 50 mA/cm2 current density. The bifunctional activity of CoNiNH was utilized for overall water splitting, which required relatively low cell potential (1.65 V) to produce 10 mA/cm2 current density. Further, the overall water splitting of CoNiNH activity was investigated in sea water also. The relatively low Tafel slope and improved charge transfer of bimetallic CoNiNH indicated the faster kinetics and supported the strong electrocatalytic activity.
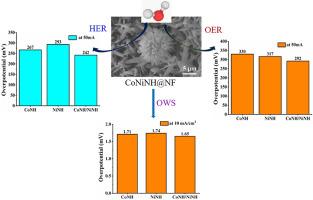
在泡沫镍上直接制备双金属氢氧化物催化剂,提高双功能和整体水分解活性
在导电泡沫镍(NF)上制备的钴-镍金属氢氧化物双金属电催化剂表现出优异的双功能电催化活性,提高了整体的水分解能力。x射线光电子能谱(XPS)分析证实了Co和Ni的存在,HR-SEM表明形成了针状纳米结构。单独的CoNH和NiNH在OER中需要330和317 mV的过电位,在HER中需要267和293 mV的过电位,才能产生50 mA/cm2的电流密度。相比之下,双金属CoNiNH需要更低的过电位(OER为292 mV, HER为242 mV)才能达到50 mA/cm2的电流密度。CoNiNH的双功能活性被用于整体的水分解,这需要相对较低的电池电位(1.65 V)来产生10 mA/cm2的电流密度。此外,还研究了海水中CoNiNH活性的整体水分裂。相对较低的Tafel斜率和更好的电荷转移表明双金属CoNiNH的动力学更快,支持了较强的电催化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


