Hydrogen production from pyrolysis of biomass components
IF 5
Q2 ENERGY & FUELS
引用次数: 0
Abstract
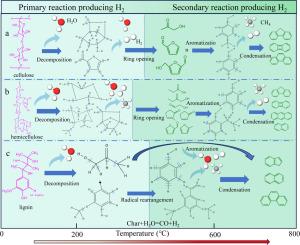
Hydrogen energy is key for the global green energy transition, and biomass thermochemical has become an important option for green hydrogen production due to its carbon neutrality advantage. Pyrolysis is the initial step of thermochemical technologies. A systematic analysis of the mechanism of H2 production from biomass pyrolysis is significant for the subsequent optimal design of efficient biomass thermochemical H2 production technologies. Biomass is mainly composed of cellulose, hemicellulose, and lignin, and differences in their physicochemical properties and structures directly affect the pyrolysis hydrogen production process. In this study, thermogravimetry-mass spectrometry-Fourier transform infrared spectroscopy (TG-MS-FTIR) was employed and fixed-bed pyrolysis experiments were conducted to systematically investigate the pyrolysis of biomass component with focusing on hydrogen production. According to the results of TG-MS-FTIR experiments, hemicellulose produced hydrogen through the breaking of C![]() H bonds in short chains and acetyl groups, as well as secondary cracking of volatiles and condensation of aromatic rings at high temperatures. Cellulose produced hydrogen through the breaking of C
H bonds in short chains and acetyl groups, as well as secondary cracking of volatiles and condensation of aromatic rings at high temperatures. Cellulose produced hydrogen through the breaking of C![]() H bonds in volatiles generated from sugar ring cleavage, along with char gasification and condensation of aromatic rings at high temperatures. Lignin produced hydrogen through ether bond cleavage, breaking of methoxy groups, as well as cleavage of phenylpropane side chains and condensation of aromatic rings at high temperatures. Results from fixed-bed pyrolysis experiments further showed that hemicellulose exhibited the strongest hydrogen production capacity, with the maximum H2 production efficiency of 6.09 mmol/g, the maximum H2 selectivity of 17.79 %, and the maximum H2 effectiveness of 59 % at 800 °C.
H bonds in volatiles generated from sugar ring cleavage, along with char gasification and condensation of aromatic rings at high temperatures. Lignin produced hydrogen through ether bond cleavage, breaking of methoxy groups, as well as cleavage of phenylpropane side chains and condensation of aromatic rings at high temperatures. Results from fixed-bed pyrolysis experiments further showed that hemicellulose exhibited the strongest hydrogen production capacity, with the maximum H2 production efficiency of 6.09 mmol/g, the maximum H2 selectivity of 17.79 %, and the maximum H2 effectiveness of 59 % at 800 °C.
生物质组分热解制氢
氢能是全球绿色能源转型的关键,生物质热化学因其碳中和优势成为绿色制氢的重要选择。热解是热化学技术的第一步。系统分析生物质热解制氢机理,对于后续优化设计高效的生物质热化学制氢技术具有重要意义。生物质主要由纤维素、半纤维素和木质素组成,其理化性质和结构的差异直接影响热解制氢过程。本研究采用热重-质谱-傅里叶变换红外光谱(TG-MS-FTIR)技术,以制氢为重点,对生物质组分的热解过程进行了系统研究。TG-MS-FTIR实验结果表明,半纤维素在高温下通过短链CH键和乙酰基的断裂、挥发物的二次裂解和芳香环的缩合产生氢气。纤维素通过糖环裂解产生的挥发物中的CH键断裂,以及高温下芳香环的焦气化和缩合产生氢。木质素在高温下通过醚键裂解、甲氧基断裂、苯基丙烷侧链裂解和芳香环缩合产生氢。固定床热解实验结果进一步表明,在800℃条件下,半纤维素的产氢能力最强,产氢效率最高为6.09 mmol/g, H2选择性最高为17.79%,H2效率最高为59%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


