Multi-omics investigation of Bisphenol A in gastrointestinal carcinogenesis: a network toxicology and molecular docking approach
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
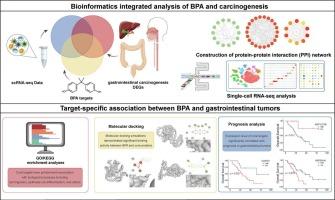
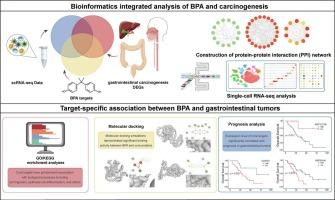
Our study aims to systematically investigate the potential carcinogenic mechanisms of bisphenol A (BPA) in three gastrointestinal tumors: intrahepatic cholangiocarcinoma (ICC), colorectal cancer (CRC), and esophageal cancer. By systematically integrating multi-omics databases, including transcriptomics and single-cell transcriptomics, we identified overlapping targets between BPA-associated molecules and tumor-related genes. Functional enrichment revealed that these targets converge on key oncogenic pathways, including cellular metabolic reprogramming (particularly glycolysis), tumor microenvironment remodeling via cancer-associated fibroblast (CAF) activation, and dysregulation of cell cycle progression. Molecular docking verified strong binding affinity between BPA and key targets. Survival analysis linked GAPDH and HSP90AA1 in ICC, CDKN1A, CEBPB, and EGR1 in CRC, and DCN and CXCL12 in esophageal cancer to poor survival rates, highlighting their potential as prognostic biomarkers. Our findings demonstrate that BPA promotes gastrointestinal carcinogenesis through disrupting energy metabolism, activating CAFs to remodel the tumor microenvironment, and enhancing cancer cell proliferation. This multi-level evidence advances the risk assessment of BPA and identifies potential targets for prevention and therapy of BPA-linked gastrointestinal cancers.
Environmental Implication.
In addition to well-documented role in promoting endocrine-related diseases, the mechanisms by which hazardous compound bisphenol A (BPA) contributes to other pathological conditions must not be overlooked. Through systematic integration of biological evidence chains, this study revealed BPA’s triple carcinogenic mechanism involving interference with cell cycle checkpoints, remodeling of tumor microenvironment stroma, and disruption of epigenetic regulation, thereby providing a novel target system for preventing environmental toxicant-induced gastrointestinal tumors.
双酚A在胃肠道癌变中的多组学研究:网络毒理学和分子对接方法
本研究旨在系统探讨双酚A (BPA)在三种胃肠道肿瘤:肝内胆管癌(ICC)、结直肠癌(CRC)和食管癌中的潜在致癌机制。通过系统整合多组学数据库,包括转录组学和单细胞转录组学,我们确定了bpa相关分子和肿瘤相关基因之间的重叠靶点。功能富集表明,这些靶点集中在关键的致癌途径上,包括细胞代谢重编程(特别是糖酵解)、通过癌症相关成纤维细胞(CAF)激活的肿瘤微环境重塑以及细胞周期进程的失调。分子对接验证了BPA与关键靶点的强结合亲和力。生存分析将ICC中的GAPDH和HSP90AA1、CRC中的CDKN1A、CEBPB和EGR1以及食管癌中的DCN和CXCL12与低生存率联系起来,突出了它们作为预后生物标志物的潜力。我们的研究结果表明,BPA通过破坏能量代谢、激活cas重塑肿瘤微环境和促进癌细胞增殖来促进胃肠道癌变。这一多层次证据促进了双酚a的风险评估,并确定了预防和治疗双酚a相关胃肠道癌症的潜在靶点。环境暗示。除了在促进内分泌相关疾病中有充分证据的作用外,有害化合物双酚A (BPA)导致其他病理状况的机制也不容忽视。本研究通过系统整合生物学证据链,揭示了BPA干扰细胞周期检查点、重塑肿瘤微环境基质、破坏表观遗传调控的三重致癌机制,为预防环境毒物致胃肠道肿瘤提供了新的靶点体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


