Pulmonary delivery of small circular RNA vaccines for influenza prevention
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lipid nanoparticles (LNPs) have played an instrumental role in the delivery of RNA therapeutics and vaccines, including the emerging class of synthetic circular RNA (circRNA). Pulmonary vaccines hold the potential to prevent various respiratory infectious diseases, such as influenza caused by influenza infection. Here, we report the pulmonary delivery of LNPs loaded with highly stable small circRNA vaccine for influenza prevention. Upon intratracheal (i.t.) instillation of circRNA LNPs into mice, circRNA was efficiently retained in the lung and was delivered to antigen-presenting cells (APCs). To elicit immune responses against influenza, we designed and synthesized a small circRNA-M2e encoding matrix protein 2 ectodomain (M2e), a highly conserved but poorly immunogenic antigen from influenza A. I.t. administration of circRNA-M2e LNPs elicited robust anti-M2e immunity in both young adult mice and 18-month-old aged mice. As a result, pulmonary delivery of LNPs loaded with circRNA-M2e vaccine protected mice from lethal influenza challenge, without significant adverse side effects. Collectively, these results demonstrate the potential of pulmonary circRNA vaccines for respiratory infectious diseases such as influenza.
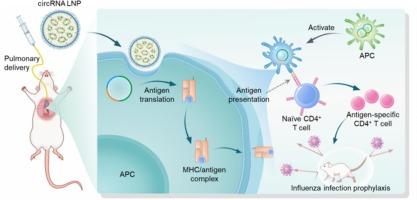
肺递送小环状RNA疫苗预防流感
脂质纳米颗粒(LNPs)在RNA疗法和疫苗的递送中发挥了重要作用,包括新兴的合成环状RNA (circRNA)。肺部疫苗有可能预防各种呼吸道传染病,例如由流感感染引起的流感。在这里,我们报道了携带高度稳定的小环状rna疫苗的LNPs的肺递送,用于预防流感。经气管内(i.t)注入环状rna LNPs后,环状rna被有效地保留在肺中,并被递送到抗原呈递细胞(APCs)。为了引发对流感的免疫应答,我们设计并合成了一种编码基质蛋白2外结构域(M2e)的小circRNA-M2e,这是一种来自流感病毒的高度保守但免疫原性较差的抗原。在年轻成年小鼠和18月龄小鼠中,给药circRNA-M2e LNPs可引发强大的抗M2e免疫。结果,肺递送携带circRNA-M2e疫苗的LNPs保护小鼠免受致命流感攻击,没有明显的不良副作用。总的来说,这些结果证明了环状rna疫苗在呼吸道传染病(如流感)中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


