Self-immolative fluorinated nanotheranostics amplifying 19F MRI signals for tumor-specific imaging and photodynamic therapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Fluorine-19 magnetic resonance imaging (19F MRI) offers distinct advantages, including background-free signal detection, quantitative analysis, and deep tissue penetration. However, its application is currently limited by challenges associated with existing 19F MRI contrast agents, such as short transverse relaxation times (T2), limited imaging sensitivity, and suboptimal biocompatibility. To overcome these limitations, a glutathione (GSH)-responsive triblock copolymer (PB7), featuring self-immolative characteristics, has been developed. In aqueous solution, PB7 can spontaneously self-assemble into a 19F MRI contrast agent (SPTF), which exhibits a long T2 relaxation time and GSH-responsive T2 prolongation. Notably, during the self-assembly process of PB7, the photosensitizer Chlorin e6 (Ce6) can be encapsulated inside of the hydrophobic domain of SPTF, resulting in the formation of a multifunctional nanotheranostic agent (Ce6@SPTF). Ce6@SPTF is able to undergo structural disintegration in response to elevated GSH levels at the tumor site, leading to the dissociation of fluorinated segments and a marked amplification of the 19F MRI signal. Concurrently, the controlled release of Ce6 generates high levels of reactive oxygen species (ROS) under laser irradiation, enabling effective in vivo ablation of breast tumors. This study presents a promising strategy to effectively combine 19F MRI with therapeutic interventions.
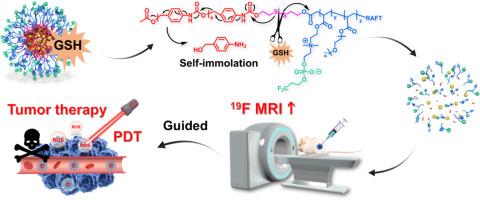
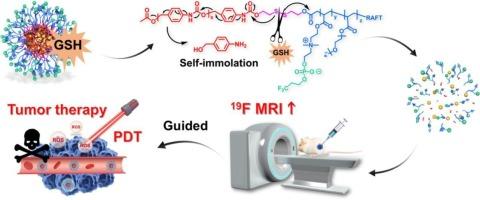
自焚氟化纳米治疗仪放大19F MRI信号用于肿瘤特异性成像和光动力治疗
氟-19磁共振成像(19F MRI)具有明显的优势,包括无背景信号检测,定量分析和深层组织渗透。然而,其应用目前受到现有19F MRI造影剂相关挑战的限制,如横向弛豫时间短(T2)、有限的成像灵敏度和不理想的生物相容性。为了克服这些限制,一种谷胱甘肽(GSH)响应的三嵌段共聚物(PB7)被开发出来,具有自燃特性。在水溶液中,PB7可以自发组装成19F MRI造影剂(SPTF),表现出较长的T2弛豫时间和gsh响应的T2延长。值得注意的是,在PB7的自组装过程中,光敏剂氯e6 (Ce6)可以被封装在SPTF的疏水结构域内,从而形成多功能纳米治疗剂(Ce6@SPTF)。Ce6@SPTF能够随着肿瘤部位GSH水平的升高而发生结构解体,导致氟化段的解离和19F MRI信号的显著放大。同时,Ce6的可控释放在激光照射下产生高水平的活性氧(ROS),使乳腺肿瘤在体内有效消融。本研究提出了一种有前景的策略,有效地将19F MRI与治疗干预相结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


