Design, synthesis, and anti-cancer evaluation of NQO1-responsive prodrug of gemcitabine
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
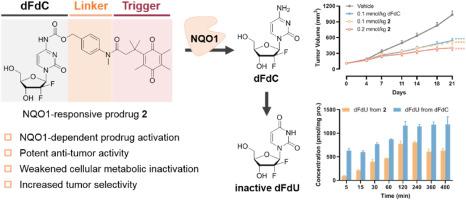
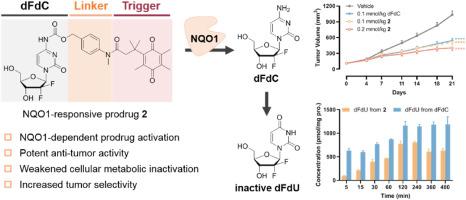
Enhancing the efficacy of gemcitabine (dFdC), a widely used nucleoside analogue in cancer therapy, through prodrug strategies to address clinical issues such as drug resistance and safety is a highly appealing and promising approach. This study focused on NQO1, a redox enzyme highly expressed in tumor cells, and designed a novel class of NQO1-responsive dFdC prodrugs. Among these prodrugs, prodrug 2 remained stable in plasma and liver/intestinal S9 fractions, releasing dFdC in an NQO1-dependent manner. Prodrug 2 demonstrated significant antitumor activity against both A549 and MCF-7 cell lines, primarily inducing S-phase arrest and apoptosis. It also inhibited tumor cell colony formation and migration. Additionally, prodrug 2 had the potential to overcome dFdC resistance and can efficiently generate dFdC within cells while producing fewer inactive metabolites (dFdU) than dFdC. Furthermore, in an A549 xenograft tumor model in mice, prodrug 2 significantly reduced tumor volume without affecting survival or body weight. Overall, our study demonstrates that an NQO1-responsive prodrug strategy can effectively enhance the antitumor properties of dFdC. The optimized prodrug 2 warrants further development as a preclinical candidate drug.
吉西他滨nqo1应答前药的设计、合成及抗癌评价
吉西他滨是一种广泛应用于癌症治疗的核苷类似物,通过前药策略来提高吉西他滨(dFdC)的疗效,以解决诸如耐药性和安全性等临床问题是一种非常有吸引力和前景的方法。本研究以肿瘤细胞中高表达的氧化还原酶NQO1为研究对象,设计了一类新型的响应NQO1的dFdC前药。在这些前药中,前药2在血浆和肝/肠S9组分中保持稳定,以nqo1依赖的方式释放dFdC。前药2对A549和MCF-7细胞系均有显著的抗肿瘤活性,主要诱导s期阻滞和凋亡。抑制肿瘤细胞集落形成和迁移。此外,前药2具有克服dFdC抗性的潜力,可以在细胞内有效地产生dFdC,同时产生的非活性代谢物(dFdU)比dFdC少。此外,在小鼠A549异种移植肿瘤模型中,前药2显著减少肿瘤体积,但不影响生存或体重。综上所述,我们的研究表明,响应nqo1的前药策略可以有效地增强dFdC的抗肿瘤特性。优化后的前药2作为临床前候选药物值得进一步开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


