Expanding the antiviral arsenal: N-arylated 1,2,4-oxadiazol-5(4H)-ones show high activity against orthopoxviruses
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
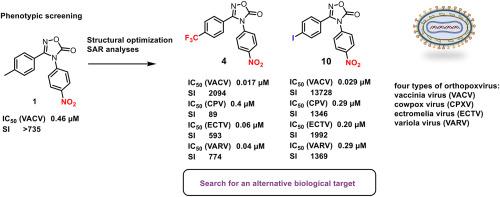
The study presents the discovery of a novel class of N-arylated 1,2,4-oxadiazol-5(4H)-ones as potent inhibitors of orthopoxviruses, including the variola virus (VARV). Through systematic structural modifications, two lead compounds, 4 (4-CF3/4-NO2) and 10 (4-I/4-NO2), demonstrated in submicromolar concentration antiviral activity against Vaccinia virus (VACV), cowpox virus (CPXV), ectromelia virus (ECTV), and VARV, with selectivity indices (SI) up to 13738. Studies of mechanisms of action, including time-of-addition experiments and molecular modeling, have shown that these compounds can target the conserved protein p37, which plays a key role in the envelope of the virus. Furthermore, bioinformatic analysis revealed potential interactions with late-stage replication proteins encoded by the A39R and C8L genes. The synthesized derivatives showed activity higher than that of Cidofovir, although they were less effective than that of Tecovirimate. This work highlights the potential of oxadiazolone-based scaffolds as broad-spectrum antipoxviral agents that meet the unmet need for therapy against emerging and re-emerging orthopoxviral threats.
扩展抗病毒武库:n -芳基化1,2,4-恶二唑-5(4H)- 1对正痘病毒具有高活性
该研究发现了一类新的n -芳基化1,2,4-恶二唑-5(4H)- 1,作为包括天花病毒(VARV)在内的正痘病毒的有效抑制剂。通过系统的结构修饰,先导化合物4 (4- cf3 /4- no2)和10 (4- i /4- no2)对牛痘病毒(VACV)、牛痘病毒(CPXV)、猪痘病毒(ECTV)和VARV具有亚微摩尔浓度的抗病毒活性,选择性指数(SI)高达13738。对作用机制的研究,包括添加时间实验和分子模型,表明这些化合物可以靶向在病毒包膜中起关键作用的保守蛋白p37。此外,生物信息学分析揭示了与A39R和C8L基因编码的后期复制蛋白的潜在相互作用。合成的衍生物活性高于西多福韦,但低于特可维美特。这项工作强调了基于恶二唑酮的支架作为广谱抗痘病毒药物的潜力,可以满足治疗新出现和再出现的正痘病毒威胁的未满足需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


