Reversible Mn3+/Mn2+ redox chemistry for high-rate aqueous manganese-ion batteries
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The Mn3+/Mn2+ redox couple is a promising candidate for high-rate energy storage scenarios owing to its high theoretical voltage and rapid redox kinetics. However, critical challenges such as Mn3+ disproportionation and shuttle effects in aqueous electrolytes significantly limit practical implementation. Herein, we propose a synergistic strategy integrating coordination optimization and interfacial confinement to achieve highly reversible Mn3+/Mn2+ redox chemistry in a mild aqueous electrolyte. Ethylenediaminetetraacetic coordinating anions are utilized to reshape the first solvation shell of Mn2+, stabilizing hydrated Mn3+ intermediates and enabling a single-electron-dominated redox pathway. A sucrose-derived molecular adsorption layer is engineered on the cathode surface through electrostatic polarity interactions, effectively suppressing Mn3+ migration into the bulk electrolyte. The well-designed Mn3+/Mn2+ cathode delivers an areal capacity of 0.36 mAh cm−2 at 4 mA cm−2 with 81 % capacity retention over 3000 cycles. As proof of concept, a high-rate aqueous Mn-ion battery is assembled by pairing the Mn3+/Mn2+ cathode with a polyimide anode, achieving a specific capacity of 104 mAh g−1 at 0.5 A g−1, exceptional rate capability, and 73.2 % capacity retention after 1000 cycles. This work unveils the directional regulation of manganese redox chemistry by a solvation-interfacial coupling mechanism, offering a design blueprint for next-generation grid-scale energy storage technologies.
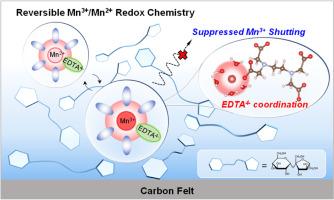
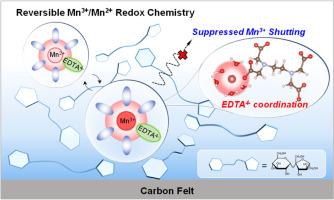
高倍率锰离子电池的可逆Mn3+/Mn2+氧化还原化学
Mn3+/Mn2+氧化还原电偶具有较高的理论电压和快速的氧化还原动力学,是高速率储能的理想选择。然而,水电解质中的Mn3+歧化和穿梭效应等关键挑战极大地限制了实际应用。在此,我们提出了一种整合配位优化和界面约束的协同策略,以在温和的水电解质中实现高度可逆的Mn3+/Mn2+氧化还原化学。利用乙二胺四乙酸配位阴离子重塑Mn2+的第一溶剂化壳层,稳定水合Mn3+中间体,实现单电子主导的氧化还原途径。通过静电极性相互作用,在阴极表面设计了蔗糖衍生的分子吸附层,有效地抑制了Mn3+向体电解质的迁移。精心设计的Mn3+/Mn2+阴极在4 mA cm - 2时提供0.36 mAh cm - 2的面积容量,在3000次循环中保持81%的容量。作为概念验证,通过将Mn3+/Mn2+阴极与聚酰亚胺阳极配对来组装高倍率水性锰离子电池,在0.5 a g - 1时实现了104 mAh g - 1的面容量,卓越的倍率能力,1000次循环后的容量保持率为73.2%。这项工作揭示了锰氧化还原化学通过溶剂-界面耦合机制的定向调节,为下一代电网规模的储能技术提供了设计蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


