A cucurbituril-based N-doped carbon nanomaterial for efficient detection and removal of Fe(CN)63−
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Fe(CN)63−, a stable cyanide complex, decomposes under alkaline or photolytic conditions to release toxic CN−, an industrially important but highly hazardous anion. To address this environmental issue, we synthesized blue-fluorescent carbon dots using cucurbit[8]uril (Q[8]) and EDTA as precursors. These carbon dots form ion associates with Fe(CN)63−, significantly altering their absorption spectrum and markedly enhancing the Resonance Rayleigh Scattering (RRS) signal, with a detection limit as low as 4.86 nM. Additionally, these nanomaterials effectively remove Fe(CN)63− from aqueous solutions. Within the concentration range of 40 to 100 μM, the removal efficiency exceeds 80 %. The Q[8]-derived framework combines selective recognition with efficient adsorption, demonstrating its potential for real-time monitoring and remediation of toxic ferricyanides in aquatic environments.
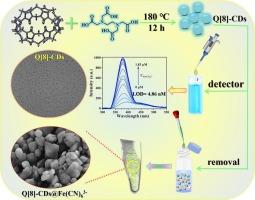
一种有效检测和去除Fe(CN)63−的瓜脲基n掺杂碳纳米材料
Fe(CN)63−是一种稳定的氰化物配合物,在碱性或光解条件下分解释放出有毒的CN−,CN−是工业上重要但非常危险的阴离子。为了解决这一环境问题,我们以瓜b[8] (Q[8])和EDTA为前体合成了蓝色荧光碳点。这些碳点与Fe(CN)63−形成离子缔合物,显著改变了它们的吸收光谱,显著增强了共振瑞利散射(RRS)信号,检测限低至4.86 nM。此外,这些纳米材料还能有效地去除水溶液中的Fe(CN)63−。在40 ~ 100 μM的浓度范围内,去除率超过80%。q[8]衍生的框架结合了选择性识别和高效吸附,显示了其在水生环境中有毒铁氰化物的实时监测和修复方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


