Constructing robust Cu and P Lewis acid-base pair sites on tubular g-C3N4 for promoting photocatalytic reduction of CO2 to CH4
IF 11.6
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
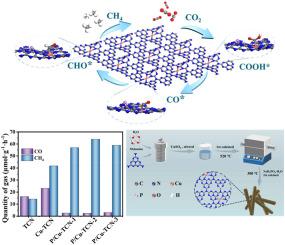
The CO2 photoreduction to high-value-added products is accompanied by a complex activation and dissociation process, and the construction of multiple active sites on photocatalysts for both CO2 reduction and H2O dissociation simultaneously is still a daunting challenge. Herein, Cu as Lewis acid (LA) sites and P as Lewis base (LB) sites were successfully modified on the surface of tubular g-C3N4 (P/Cu-TCN) to improve the performance of CO2 photoreduction to CH4 with H2O as a proton donor. The production of CH4 is as high as 63.95 μmol g−1 h−1 over optimal P/Cu-TCN photocatalyst with an outstanding selectivity. The performance is much higher than those of the reported g–C3N4–based photocatalytic systems. Experimental results combined with theoretical simulation results show that the electrophilic Cu (Lewis acid) centers induce the activation of the C![]() O bond in CO2, while electron-enriched P (Lewis base) sites enhance the adsorption of pure water molecules and subsequent proton transfer processes. The incorporation of Cu and P acid-base pairs not only mitigates the elevated Gibbs free energy barrier associated with C
O bond in CO2, while electron-enriched P (Lewis base) sites enhance the adsorption of pure water molecules and subsequent proton transfer processes. The incorporation of Cu and P acid-base pairs not only mitigates the elevated Gibbs free energy barrier associated with C![]() O bond cleavage during CO2 photoreduction and facilitates the overall proton-coupled electron transfer kinetics, but also suppresses the recombination of photogenerated charge carriers while enhancing charge transport efficiency. All of these together improve the catalytic efficiency of the CO2-to-CH4 transformation. This study offers research suggestions for the modification of g-C3N4 to enhance CO2 reduction.
O bond cleavage during CO2 photoreduction and facilitates the overall proton-coupled electron transfer kinetics, but also suppresses the recombination of photogenerated charge carriers while enhancing charge transport efficiency. All of these together improve the catalytic efficiency of the CO2-to-CH4 transformation. This study offers research suggestions for the modification of g-C3N4 to enhance CO2 reduction.
在管状g-C3N4上构建稳健的Cu和P Lewis酸碱对位点,促进CO2光催化还原为CH4
CO2光还原制取高附加值产品是一个复杂的活化和解离过程,在光催化剂上构建多个同时用于CO2还原和H2O解离的活性位点仍然是一个艰巨的挑战。本文成功地在管状g-C3N4 (P/Cu- tcn)表面修饰了Cu作为路易斯酸(LA)位点和P作为路易斯碱(LB)位点,以提高H2O作为质子供体的CO2光还原成CH4的性能。最佳P/Cu-TCN光催化剂的CH4产率高达63.95 μmol g−1 h−1,具有良好的选择性。性能远高于已有报道的基于g - c3n4的光催化体系。实验结果结合理论模拟结果表明,亲电性的Cu (Lewis酸)中心诱导CO2中CO键的活化,而富电子的P (Lewis碱)位点增强了纯水分子的吸附和随后的质子转移过程。Cu和P酸碱对的加入不仅减轻了CO2光还原过程中CO键断裂引起的吉布斯自由能垒升高,促进了整体质子耦合电子传递动力学,而且还抑制了光生载流子的重组,提高了电荷传输效率。这些共同提高了CO2-to-CH4转化的催化效率。本研究为g-C3N4的改性提高CO2的还原能力提供了研究建议。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


