TRIM39 reinforces E2-ESR1 signaling through SUMOylation of ESR1 to hinder the progression of aortic dissection
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background and aims
Aortic dissection (AD) is one of the most dangerous and tricky diseases in the field of cardiovascular surgery, severely affecting public health. Recent studies have found that SUMOylation is linked to the pathogenesis of cardiovascular diseases. However, we know very little about the molecular mechanisms of SUMOylation in AD.
Methods
Clinical samples of AD and normal aorta were collected for transcriptome sequencing analysis. qPCR and Western blot were utilized to examine the expression of TRIM39 in clinical samples. AD mouse model and cell model were constructed to test the effect of TRIM39 overexpression on the progression of AD. With the help of bioinformatics analysis tools UBIBROWSER, BIOGRID, and GPS-SUMO, the substrate protein ESR1 of TRIM39 was predicted. Combined with CO-IP, we verified whether TRIM39 SUMOylated ESR1 and how SUMOylation affected ESR1 protein.
Results
TRIM39 was greatly downregulated in AD samples, and ESR1 is a downstream target protein of TRIM39. In vivo and in vitro experiments revealed that TRIM39 overexpression alleviated the phenotype of AD by changing the contractile phenotype and cell function of aortic smooth muscle cells, and this process depended on the activation of ESR1 by E2. Mechanistically, TRIM39 mediated the SUMOylation of ESR1, thereby enhancing its protein stability and strengthening E2-ESR1 signaling.
Conclusions
TRIM39 modifies ESR1 through SUMOylation and enhances its stability, facilitating E2-ESR1 signaling and alleviating AD progression.
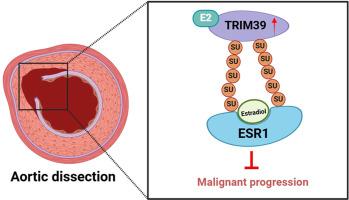
TRIM39通过ESR1的SUMOylation增强E2-ESR1信号,从而阻碍主动脉夹层的进展
背景与目的主动脉夹层(AD)是心血管外科领域最危险、最棘手的疾病之一,严重影响公众健康。最近的研究发现,SUMOylation与心血管疾病的发病机制有关。然而,我们对AD中SUMOylation的分子机制知之甚少。方法收集AD和正常主动脉临床标本,进行转录组测序分析。应用qPCR和Western blot检测TRIM39在临床样品中的表达。建立AD小鼠模型和细胞模型,检测TRIM39过表达对AD进展的影响。利用生物信息学分析工具UBIBROWSER、BIOGRID和GPS-SUMO预测TRIM39的底物蛋白ESR1。结合CO-IP,我们验证了TRIM39是否SUMOylation ESR1以及SUMOylation如何影响ESR1蛋白。结果trimm39在AD样品中显著下调,ESR1是TRIM39的下游靶蛋白。体内和体外实验表明,TRIM39过表达通过改变主动脉平滑肌细胞的收缩表型和细胞功能来减轻AD的表型,而这一过程依赖于E2对ESR1的激活。机制上,TRIM39介导ESR1的SUMOylation,从而增强其蛋白稳定性,增强E2-ESR1信号传导。结论strim39通过SUMOylation修饰ESR1,增强其稳定性,促进E2-ESR1信号通路,缓解AD进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis
医学-外周血管病
CiteScore
9.80
自引率
3.80%
发文量
1269
审稿时长
36 days
期刊介绍:
Atherosclerosis has an open access mirror journal Atherosclerosis: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atherosclerosis brings together, from all sources, papers concerned with investigation on atherosclerosis, its risk factors and clinical manifestations. Atherosclerosis covers basic and translational, clinical and population research approaches to arterial and vascular biology and disease, as well as their risk factors including: disturbances of lipid and lipoprotein metabolism, diabetes and hypertension, thrombosis, and inflammation. The Editors are interested in original or review papers dealing with the pathogenesis, environmental, genetic and epigenetic basis, diagnosis or treatment of atherosclerosis and related diseases as well as their risk factors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


