Development of novel 18F-labelled FAP-targeting tracers with improved pharmacokinetics: From preclinical optimization to clinical translation
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
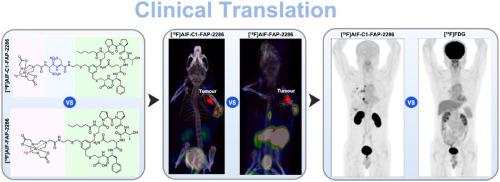
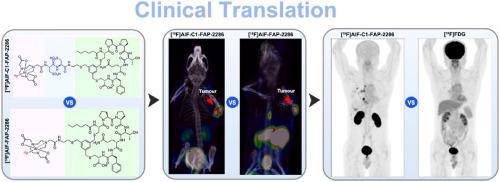
Fibroblast activation protein (FAP) has emerged as a promising theranostic target in malignancies. Although numerous radiolabelled FAP-targeting tracers have been clinically used for tumour imaging, the development of 18F-labelled tracers remains an unmet clinical need. This study synthesized two NOTA-conjugated FAP-2286 derivatives, modified with cysteic acid (C1) and/or tranexamic acid (C2) moieties. Both tracers exhibited >96 % radiochemical purity with molar activities >14.3 GBq/μmol. High stability and FAP specificity were demonstrated in vitro. [18F]AlF-C1-FAP-2286 demonstrated superior pharmacokinetics with higher tumour-to-kidney (2.19 ± 0.74 vs 1.54 ± 0.72) and tumour-to-liver ratios (18.32 ± 6.32 vs 13.74 ± 4.61) compared to [18F]AlF-FAP-2286. Clinical PET imaging with [18F]AlF-C1-FAP-2286 enabled the clear delineation of the recurrent lesion at the surgical site and metastatic tumours, demonstrating favourable imaging characteristics, high tumour uptake, and contrast quality. Overall, [18F]AlF-C1-FAP-2286 exhibited optimized pharmacokinetic properties and enhanced tumour contrast. The above findings support its potential as a promising 18F-labelled FAP-targeting tracer, warranting its further exploration in clinical applications.
Trial registration
ChiCTR2400090727. Registered October 12, 2024
改进药代动力学的新型18f标记fap靶向示踪剂的开发:从临床前优化到临床转化
成纤维细胞活化蛋白(FAP)已成为一种有前景的恶性肿瘤治疗靶点。尽管许多放射性标记的fap靶向示踪剂已被临床用于肿瘤成像,但18f标记示踪剂的开发仍未满足临床需求。本研究合成了两个nota共轭的FAP-2286衍生物,分别用半胱酸(C1)和/或氨甲环酸(C2)修饰。两种示踪剂的放射化学纯度为96%,摩尔活性为14.3 GBq/μmol。体外稳定性和FAP特异性高。与[18F]AlF-FAP-2286相比,[18F]AlF-C1-FAP-2286表现出更好的药代动力学,肿瘤与肾脏的比值(2.19±0.74 vs 1.54±0.72)和肿瘤与肝脏的比值(18.32±6.32 vs 13.74±4.61)更高。使用[18F]AlF-C1-FAP-2286进行临床PET成像,可以清晰地描绘手术部位的复发病变和转移性肿瘤,显示出良好的成像特征、高肿瘤摄取和对比度质量。总体而言,[18F]AlF-C1-FAP-2286表现出优化的药代动力学特性和增强的肿瘤对比。上述发现支持其作为一种有前途的18f标记的fap靶向示踪剂的潜力,值得其在临床应用中的进一步探索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


