Nepenthes-Inspired Slippery Liquid-Infused Porous Surfaces on Gold Coatings with Dendritic Fluorinated Gold Nanosheets for Long-Lasting Anticoagulation and Antimicrobial Applications
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The construction of perfluoropolyether (PFPE) slippery liquid-infused porous surfaces (SLIPS) on gold coatings is one of the most effective strategies for bestowing anticoagulation and antimicrobial properties on the material. However, the poor chemical affinity between fluorinated porous precursors and gold substrates causes the agglomeration of nanostructures, resulting in uneven nanoporous morphology and accelerating lubricant leakage. Simultaneously, the weak interfacial adhesion between the nanostructures and the substrate may lead to the detachment of nanostructures under blood circulation. Herein, fluorinated gold nanoporous structures exhibiting uniform morphology were tethered on gold coatings via metallic bonding. The vertically aligned dendritic gold frameworks were prepared via in situ chloroauric acid disproportionation, which avoided agglomeration and served as the nanoporous structure. Fluorinated alkyl chains were covalently grafted onto the nanostructures with no considerable influence on morphology. The nanostructures showed no severe damage after 20 min of ultrasonication and 14 days of saline immersion. No significant lubricant leakage was detected after 24 h of in vitro blood circulation. The SLIPS significantly resisted the adhesion of thrombocytes and plasma proteins and suppressed biofilm formation. Histological evaluation showed significantly reduced inflammatory responses and cell infiltration at surgical margins.
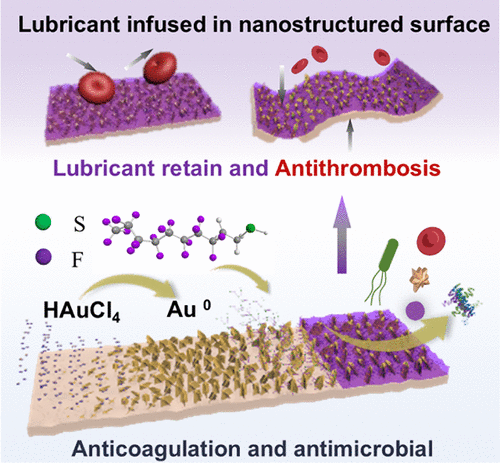
树突状氟化金纳米片的金涂层上受新花启发的光滑液体注入多孔表面,用于长效抗凝和抗菌应用
在金涂层上构建全氟聚醚(PFPE)光滑的液体注入多孔表面(slip)是赋予材料抗凝和抗菌性能的最有效策略之一。然而,氟化多孔前驱体与金衬底之间的化学亲和力较差,导致纳米结构团聚,导致纳米孔形态不均匀,加速润滑剂泄漏。同时,纳米结构与基质之间的界面粘附较弱,可能导致纳米结构在血液循环下脱离。本文通过金属键将具有均匀形貌的氟化金纳米孔结构系在金涂层上。采用原位氯金酸歧化法制备了垂直排列的树枝状金骨架,避免了团聚,具有纳米孔结构。氟化烷基链被共价接枝到纳米结构上,对形貌没有太大的影响。超声作用20 min,盐水浸泡14 d后,纳米结构未见明显损伤。体外血液循环24 h后未发现明显的润滑油泄漏。slip显著抵抗血小板和血浆蛋白的粘附,抑制生物膜的形成。组织学评估显示手术边缘的炎症反应和细胞浸润明显减少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


