Lysine-polydopamine nanoparticles for ameliorating acidic microenvironment and neuroprotection in spinal cord injury repair
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-03
DOI:10.1016/j.bioadv.2025.214479
引用次数: 0
Abstract
Spinal cord injury (SCI) is exacerbated by the formation of an acidic microenvironment and extensive neuronal loss, both of which contribute to poor functional recovery. To address this, we developed lysine-polydopamine nanoparticles (Lys-PDA) as a multifunctional therapeutic platform for SCI. Lysine, a naturally occurring amino acid, possesses weak alkalinity and neuroprotective properties, but suffers from poor in vivo stability and non-specific distribution. Polydopamine (PDA), a biocompatible polymer with inherent antioxidant and anti-inflammatory capabilities, was employed as a nanocarrier to enhance lysine delivery and local retention. In vitro and in vivo assessments confirmed that Lys-PDA exhibit excellent biocompatibility and enable sustained release of lysine for up to three days. Upon administration into SCI mice, Lys-PDA significantly neutralized the acidic lesion milieu, with pH values increasing by approximately 0.33 units—approaching levels observed in uninjured spinal tissue. After 28 days of treatment, Lys-PDA markedly reduced neuronal apoptosis, suppressed reactive oxygen species (ROS) and lactate accumulation, and significantly improved hindlimb motor function. Behavioral analyses demonstrated a substantial increase in Basso Mouse Scale (BMS) scores from 0.0 to 5.8, alongside a corresponding increase in hindlimb stride length from 2.0 cm to 4.3 cm. Collectively, these findings suggest that Lys-PDA not only modulate the hostile post-injury microenvironment but also promote neuroprotection and functional recovery, positioning them as a promising candidate for SCI repair.
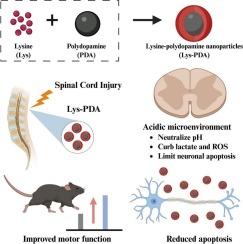
赖氨酸-聚多巴胺纳米颗粒改善酸性微环境和脊髓损伤修复中的神经保护作用。
酸性微环境的形成和广泛的神经元损失加剧了脊髓损伤(SCI),这两者都导致功能恢复不良。为了解决这个问题,我们开发了赖氨酸-聚多巴胺纳米颗粒(赖氨酸- pda)作为脊髓损伤的多功能治疗平台。赖氨酸是一种天然存在的氨基酸,具有弱碱性和神经保护作用,但体内稳定性差,分布无特异性。聚多巴胺(PDA)是一种生物相容性聚合物,具有固有的抗氧化和抗炎能力,被用作纳米载体来增强赖氨酸的递送和局部保留。体外和体内评估证实,赖氨酸- pda具有良好的生物相容性,并能持续释放赖氨酸长达三天。在给药于脊髓损伤小鼠后,Lys-PDA显著中和了酸性损伤环境,pH值增加了约0.33个单位,接近未损伤脊髓组织中观察到的水平。治疗28 d后,Lys-PDA显著减少神经元凋亡,抑制活性氧(ROS)和乳酸积累,显著改善后肢运动功能。行为学分析表明,小鼠的BMS评分从0.0增加到5.8,后肢跨步长度从2.0厘米增加到4.3厘米。综上所述,这些发现表明Lys-PDA不仅可以调节损伤后的不良微环境,还可以促进神经保护和功能恢复,使其成为有希望修复脊髓损伤的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


