Glucose metabolism in anterior pituitary adenomas
IF 2.9
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
Glucose metabolism alterations are frequently observed in patients with secretory pituitary adenomas. The most commonly secreted hormones in these tumors include prolactin, growth hormone (GH), adrenocorticotropic hormone (ACTH), and thyroid-stimulating hormone (TSH), all of which can disrupt glucose homeostasis through distinct pathophysiological mechanisms. Prolactin stimulates pancreatic β-cell proliferation, enhances insulin gene transcription, increases intracellular insulin content, and augments glucose-induced insulin secretion. GH promotes lipolysis and hepatic glucose production while impairing peripheral glucose uptake, contributing to insulin resistance. ACTH, via excess glucocorticoids, reduces GLUT-4 translocation, enhances gluconeogenesis and proteolysis, and inhibits glycogen synthesis. Excessive TSH leads to increased T3 and T4 production, which in turn stimulate glucose uptake in muscle and enhance both glycolysis and gluconeogenesis. This review summarizes the current understanding of how hormone hypersecretion in pituitary adenomas contributes to glucose metabolism dysregulation.
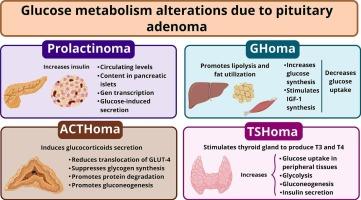
垂体前腺瘤的糖代谢。
糖代谢改变常见于垂体腺瘤患者。这些肿瘤中最常见的分泌激素包括催乳素、生长激素(GH)、促肾上腺皮质激素(ACTH)和促甲状腺激素(TSH),它们都可以通过不同的病理生理机制破坏葡萄糖稳态。催乳素刺激胰腺β细胞增殖,增强胰岛素基因转录,增加细胞内胰岛素含量,增加葡萄糖诱导的胰岛素分泌。生长激素促进脂肪分解和肝脏葡萄糖生成,同时损害外周葡萄糖摄取,促进胰岛素抵抗。ACTH通过过量的糖皮质激素,减少GLUT-4易位,增强糖异生和蛋白水解,抑制糖原合成。过量的TSH导致T3和T4生成增加,这反过来刺激肌肉的葡萄糖摄取,增强糖酵解和糖异生。本文综述了目前对垂体腺瘤中激素高分泌如何导致糖代谢失调的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinica Chimica Acta
医学-医学实验技术
CiteScore
10.10
自引率
2.00%
发文量
1268
审稿时长
23 days
期刊介绍:
The Official Journal of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Clinica Chimica Acta is a high-quality journal which publishes original Research Communications in the field of clinical chemistry and laboratory medicine, defined as the diagnostic application of chemistry, biochemistry, immunochemistry, biochemical aspects of hematology, toxicology, and molecular biology to the study of human disease in body fluids and cells.
The objective of the journal is to publish novel information leading to a better understanding of biological mechanisms of human diseases, their prevention, diagnosis, and patient management. Reports of an applied clinical character are also welcome. Papers concerned with normal metabolic processes or with constituents of normal cells or body fluids, such as reports of experimental or clinical studies in animals, are only considered when they are clearly and directly relevant to human disease. Evaluation of commercial products have a low priority for publication, unless they are novel or represent a technological breakthrough. Studies dealing with effects of drugs and natural products and studies dealing with the redox status in various diseases are not within the journal''s scope. Development and evaluation of novel analytical methodologies where applicable to diagnostic clinical chemistry and laboratory medicine, including point-of-care testing, and topics on laboratory management and informatics will also be considered. Studies focused on emerging diagnostic technologies and (big) data analysis procedures including digitalization, mobile Health, and artificial Intelligence applied to Laboratory Medicine are also of interest.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


