Boosting biocompatibility and minimizing inflammation in electrospun polyvinylidene fluoride (PVDF) cardiac patches through optimized low-pressure plasma treatment
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-02
DOI:10.1016/j.bioadv.2025.214488
引用次数: 0
Abstract
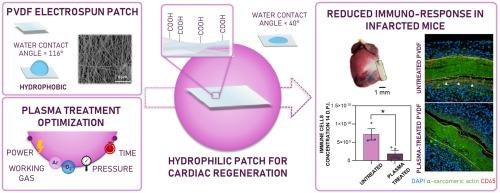
Tailoring surface characteristics is key to guiding scaffold interaction with the biological environment, promoting successful biointegration while minimizing immune responses and inflammation.
In cardiac tissue engineering, polyvinylidene fluoride (PVDF) is a material of choice for its intrinsic piezoelectric properties, which can be enhanced through electrospinning, also enabling the fabrication of nanofibrous structures mimicking native tissue. However, the inherent hydrophobicity of PVDF can hinder its integration with biological tissues.
To overcome this limitation, electrospun PVDF patches were subjected to radio-frequency low-pressure O2 plasma treatment to enhance surface hydrophilicity and overall biocompatibility. A systematic experimental study identified optimal parameters, revealing that higher gas content and prolonged exposure are preferable to high power levels, which deteriorate the patch's morphological and mechanical properties.
X-ray photoelectron spectroscopy confirmed the formation of oxygen-containing surface groups, resulting in the patch's superhydrophilicity. Preservation of the fibrous nanostructure and electroactive phase content was verified using scanning electron microscopy and infrared spectroscopy combined with differential scanning calorimetry, respectively. The optimized plasma treatment maintained the patch's elasticity and demonstrated long-term stability for up to 3 months.
In vitro biocompatibility was assessed through indirect and direct tests using AC16 human cardiomyocytes and neonatal human dermal fibroblasts, revealing good cell viability, adhesion, and spreading over 7-days. Finally, plasma-treated patches demonstrated strong adhesion to the myocardial tissue and exhibited markedly reduced inflammatory response compared to the untreated controls, as shown by decreased CD45+ immune cell infiltration around the patch implanted in infarcted mice, highlighting the surface treatment's effectiveness in enhancing in vivo biocompatibility.
通过优化低压血浆处理提高聚偏氟乙烯(PVDF)心脏贴片的生物相容性并减少炎症
定制表面特征是指导支架与生物环境相互作用的关键,促进成功的生物整合,同时最大限度地减少免疫反应和炎症。在心脏组织工程中,聚偏氟乙烯(PVDF)因其固有的压电特性而成为首选材料,这种特性可以通过静电纺丝增强,也可以制造模拟天然组织的纳米纤维结构。然而,PVDF固有的疏水性会阻碍其与生物组织的结合。为了克服这一限制,静电纺PVDF贴片进行了射频低压氧等离子体处理,以提高表面亲水性和整体生物相容性。一项系统的实验研究确定了最佳参数,表明高气体含量和长时间暴露比高功率水平更可取,因为高功率水平会破坏贴片的形态和力学性能。x射线光电子能谱证实了含氧表面基团的形成,导致了贴片的超亲水性。利用扫描电镜和红外光谱结合差示扫描量热法分别验证了纤维纳米结构和电活性相含量的保存。优化后的等离子体处理保持了贴片的弹性,并表现出长达3个月的长期稳定性。通过AC16人心肌细胞和新生儿人真皮成纤维细胞的间接和直接试验评估体外生物相容性,显示出良好的细胞活力、粘附性和7天内的扩散。最后,与未处理的对照组相比,经血浆处理的贴片与心肌组织具有较强的粘附性,炎症反应明显减少,这表明在梗死小鼠中植入的贴片周围CD45+免疫细胞浸润减少,突出了表面处理在增强体内生物相容性方面的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


