Isothermal one-pot RPA-CRISPR/Cas12b assay for rapid and highly sensitive detection of Brucella spp
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
As a zoonotic disease caused by Brucella species, brucellosis requires rapid, sensitive, and precise diagnostic approaches to effectively curb its epidemiological spread. Herein, we developed a novel isothermal one-pot recombinase polymerase amplification (RPA)-CRISPR/Cas12b (IOPR-Cas12b) assay targeting the conserved region of the Brucella spp.-specific BCSP31 gene sequence. This assay is integrated with real-time fluorescence detection and lateral flow biosensor (LFB), recognized as promising tools for Point-of-care testing (POCT) molecular diagnostics. A BCSP31-targeted IOPR-Cas12b assay was developed through systematic design and screening of RPA primers and guide RNA (gRNA), and optimization of reaction conditions. The IOPR-Cas12b achieved maximum efficiency at 43 °C for 40 min with real-time fluorescence and LFB readout methods. The system exhibited remarkable sensitivity with limits of detection (LOD) of 3.65 × 101 copies/reaction (real-time fluorescence) and 3.65 × 102 copies/reaction (LFB), while maintaining strict specificity by showing no cross-reactivity with 19 non-target bacterial species. Clinical validation using 61 samples demonstrated 100 % concordance between the IOPR-Cas12b assay and reference methods (culture and conventional PCR). The entire detection workflow was accomplished within 40 min through real-time fluorescence monitoring. Furthermore, the incorporation of visual LFB detection eliminated the reliance on sophisticated instrumentation, enhancing its suitability for field-deployable POCT applications. The developed one-pot platform combining IOPR-Cas12b with dual-mode detection (real-time fluorescence/LFB) offers a rapid, sensitive, and highly specific solution, providing a highly competitive technical means for brucellosis screening and prevention.
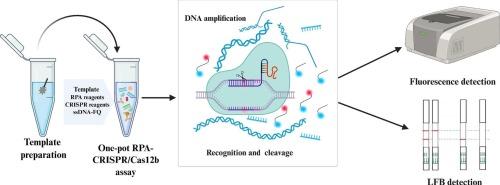
一锅等温RPA-CRISPR/Cas12b快速高灵敏度检测布鲁氏菌
作为一种由布鲁氏菌引起的人畜共患疾病,布鲁氏菌病需要快速、灵敏和精确的诊断方法,以有效遏制其流行病学传播。在此,我们开发了一种新的等温一锅重组酶聚合酶扩增(RPA)-CRISPR/Cas12b (IOPR-Cas12b)检测方法,靶向布鲁氏菌特异性BCSP31基因序列的保守区域。该检测与实时荧光检测和横向流动生物传感器(LFB)相结合,被认为是即时检测(POCT)分子诊断的有前途的工具。通过系统设计筛选RPA引物和gRNA,优化反应条件,建立以bcsp31为靶点的IOPR-Cas12b检测方法。使用实时荧光和LFB读出方法,IOPR-Cas12b在43°C下40 min达到最高效率。检测限(LOD)为3.65 × 101拷贝/反应(实时荧光)和3.65 × 102拷贝/反应(LFB),同时与19种非靶菌无交叉反应,保持了严格的特异性。61个样本的临床验证表明,IOPR-Cas12b检测与参考方法(培养和传统PCR)之间的一致性为100%。通过实时荧光监测,整个检测流程在40分钟内完成。此外,结合视觉LFB检测消除了对复杂仪器的依赖,增强了其在现场部署POCT应用中的适用性。所开发的IOPR-Cas12b与双模检测(实时荧光/LFB)相结合的一锅式平台提供了快速、灵敏、高特异性的解决方案,为布鲁氏菌病的筛查和预防提供了极具竞争力的技术手段。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


