2D copper metal-organic framework as an electrocatalyst for the highly efficient electrochemical reduction of low concentration nitrate/nitrite-to-ammonia
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The accumulation of nitrate/nitrite (NOx–) in wastewater poses a threat on ecological safety. Electrocatalytic NOx– reduction to ammonia (NOx–RR) is feasible to realize green ammonia production. This work developed a 2D copper metal-organic framework of Cu-BDC (H2BDC = 1,4-benzene dicarboxylic acid) as an efficient electrocatalyst for NOx–RR, exhibiting high-performance with the NH3 yield of 71.20 μmol‧h−1‧cm−2 and a Faraday efficiency of 80.9 % at −0.746 V (vs. RHE) for NO2−RR, and the NH3 yield of 130.16 μmol‧h−1‧cm−2 and a Faraday efficiency of 67.42 % at −0.946 V (vs. RHE) for NO3−RR. Isotope labelled 14NO3− and 15NO3− produced 14NH4+ and 15NH4+, supporting the formation of NH3 comes from the NO3−RR. In three NOx–RR cycles, all NH3 yields and Faraday efficiencies show a slight change, demonstrating an excellent electrocatalytic stability in NOx–RR. Our work provides a new material platform for the elimination of nitrate and nitrite through electrocatalytic reduction.
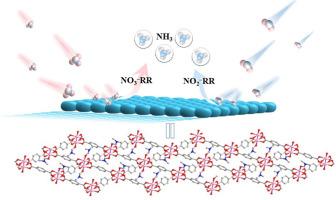
二维铜金属有机骨架电催化剂用于低浓度硝酸盐/亚硝酸盐高效电化学还原制氨
硝酸盐/亚硝酸盐(NOx -)在废水中的积累对生态安全构成威胁。电催化NOx还原制氨(NOx - rr)是实现绿色制氨的可行方法。本文开发了一种二维铜金属-有机骨架Cu-BDC (H2BDC = 1,4-苯二甲酸)作为NOx-RR的高效电催化剂,在- 0.746 V(相对于RHE)下,NO2 - RR的NH3产率为71.20 μmol·h−1·cm−2,法拉第效率为80.9%;在- 0.946 V(相对于RHE)下,NO3 - RR的NH3产率为130.16 μmol·h−1·cm−2,法拉第效率为67.42%。标记为14NO3−和15NO3−的同位素产生14NH4+和15NH4+,支持NH3的形成来自NO3−RR。在三次NOx-RR循环中,NH3产率和法拉第效率均略有变化,表明NOx-RR具有良好的电催化稳定性。本研究为电催化还原去除硝酸盐和亚硝酸盐提供了新的材料平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


