One-pot non-isocyanate urethane synthesis via visible light-activated Curtius rearrangement
IF 6.3
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Curtius rearrangement is a highly versatile and powerful synthetic strategy for converting acyl azides into isocyanates. Herein, we introduce a visible-light-induced non-isocyanate method as an innovative ligation approach for urethane linkage formation, enabling the photochemical in situ generation of isocyanates under mild conditions. We design conjugated acyl azide molecules and successfully integrate these into diverse ligation processes, showcasing their versatility in small molecule synthesis, polymer chain-end functionalization, and surface modification of both inorganic and organic substrates. The resulting small molecules and materials were comprehensively characterized using nuclear magnetic resonance (NMR), attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), ultraviolet–visible (UV–Vis) and fluorescence spectroscopy, contact angle (CA) measurements, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). Critically, density functional theory (DFT) calculations provided molecular-level insights into the reaction mechanism, revealing how electronic effects influence the initiation efficiency of acyl azides. Our simple yet highly efficient visible-light-driven ligation strategy paves the way for new applications in the fabrication of complex macromolecular architectures, advanced biomaterials, as well as hydrogel networks.
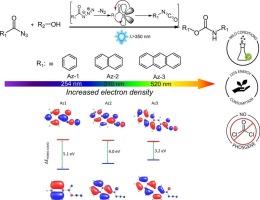
可见光活化Curtius重排法一锅法合成非异氰酸酯聚氨酯
Curtius重排是一种将酰基叠氮化物转化为异氰酸酯的高度通用和强大的合成策略。在此,我们介绍了一种可见光诱导非异氰酸酯方法,作为一种创新的氨基甲酸乙酯连接方法,使异氰酸酯在温和条件下的光化学原位生成成为可能。我们设计了共轭酰基叠氮化物分子,并成功地将它们整合到不同的连接过程中,展示了它们在小分子合成、聚合物链端功能化以及无机和有机底物表面修饰方面的多功能性。利用核磁共振(NMR)、衰减全反射-傅里叶变换红外光谱(ATR-FTIR)、紫外-可见(UV-Vis)和荧光光谱、接触角(CA)测量、扫描电子显微镜(SEM)和透射电子显微镜(TEM)对所得小分子和材料进行了全面表征。重要的是,密度泛函理论(DFT)计算提供了分子水平上对反应机理的洞察,揭示了电子效应如何影响酰基叠氮化物的引发效率。我们简单而高效的可见光驱动连接策略为复杂大分子结构、先进生物材料以及水凝胶网络的制造铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


