Novel dual responsive embelin fabricated ZnO nanomaterials amplify DNA damage and induce apoptosis via pERK1/2/p53 pathway in pancreatic ductal adenocarcinoma
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-23
DOI:10.1016/j.bioadv.2025.214470
引用次数: 0
Abstract
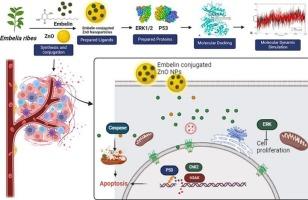
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with poor prognosis and chemoresistance. Nano-bioconjugates, due to their enhanced surface-to-volume ratio, offer significant potential in cancer therapy. In this study, we synthesized ZnO nanoparticles (NPs) using solution combustion method and exhibited a particle size range of 20–70 nm as confirmed by TEM analysis. These NPs were conjugated with embelin, a natural benzoquinone compound. Successful conjugation was confirmed using FTIR spectroscopy. Structural and morphological characteristics of the conjugates were confirmed using XRD, SEM, TEM, FTIR, EDS color mapping. Embelin conjugated ZnO NPs (Emb-ZnO NPs) were evaluated against PDAC cell lines (PANC-1 and MIA PaCa-2). The nanoconjugates showed significant cytotoxicity compared to individual Embelin and ZnO NPs, with IC50 values of 7.05 ± 0.96 μg/ml (PANC-1) and 8.66 ± 1.46 μg/ml (MIA PaCa-2). Emb-ZnO NPs exhibited tumoricidal effects in clonogenic and migration assays. Fluorescent staining revealed disrupted cellular architecture and significant apoptosis. Immunoblot analysis exhibits deregulation of key pathways, including amplified expression of γ-H2AX (88.48 %), indicative of DNA damage. Concurrently, elevated levels of pChk2 (68.07 %), p53 (89.34 %), and caspase-3 (26.67 %) promote Cell cycle halting and programmed cell death triggered by genomic instability. Conversely, reduced pERK1/2 (53.77 %) expression suggested inhibition of the MAPK pathway by Emb-ZnO NPs. Additionally, the formulation inhibited neovascularization in the CAM model, indicating anti-angiogenic potential. Molecular dynamics simulations of p53 and pERK1/2 aligned with in vitro results. In conclusion, Emb-ZnO NPs is a promising therapeutic candidate for PDAC and other cancers.
新型双响应性血管栓塞制备ZnO纳米材料在胰腺导管腺癌中通过pERK1/2/p53通路放大DNA损伤并诱导细胞凋亡
胰腺导管腺癌(Pancreatic ductal adencarcinoma, PDAC)是一种预后差、耐药的高侵袭性肿瘤。纳米生物偶联物由于其增强的表面体积比,在癌症治疗中提供了巨大的潜力。在本研究中,我们采用溶液燃烧法合成了ZnO纳米颗粒(NPs),并通过TEM分析证实其粒径范围为20-70 nm。这些NPs与天然苯醌类化合物栓塞蛋白结合。用FTIR光谱证实了成功的偶联。通过XRD、SEM、TEM、FTIR、EDS等彩色图对共轭物的结构和形态特征进行了表征。研究了Embelin偶联ZnO NPs (Emb-ZnO NPs)对PDAC细胞株(PANC-1和MIA PaCa-2)的作用。与单个Embelin和ZnO NPs相比,纳米偶联物显示出显著的细胞毒性,IC50值为7.05 ± 0.96 μg/ml (PANC-1)和8.66 ± 1.46 μg/ml (MIA PaCa-2)。Emb-ZnO NPs在克隆生成和迁移实验中显示出杀肿瘤作用。荧光染色显示细胞结构破坏和明显的凋亡。免疫印迹分析显示关键通路的失调,包括γ-H2AX的表达扩增(88.48 %),表明DNA损伤。同时,pChk2(68.07 %)、p53(89.34 %)和caspase-3(26.67 %)水平升高可促进基因组不稳定性引发的细胞周期停止和程序性细胞死亡。相反,pERK1/2表达降低(53.77 %)表明Emb-ZnO NPs抑制了MAPK通路。此外,在CAM模型中,该配方抑制新血管形成,表明抗血管生成潜力。p53和pERK1/2的分子动力学模拟与体外结果一致。总之,Emb-ZnO NPs是治疗PDAC和其他癌症的有希望的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


