Photothermal-triggered NO-releasing nanofiber membrane mitigates intervertebral disc degeneration via inflammation inhibition and matrix stabilization
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Intervertebral disc–related low back pain represents a major source of chronic pain. Due to the inflammatory microenvironment and impaired extracellular matrix (ECM) synthesis in intervertebral disc degeneration (IVDD), annulus fibrosus (AF) injuries have limited healing capacity. Effective AF repair thus requires modulation of the inflammatory state, promotion of ECM deposition, and enhancement of cellular migration. In this study, we developed a multifunctional photothermal nanofibrous membrane system by electrospinning biocompatible chitosan (CS) and polyvinyl alcohol (PVA) integrated with the photosensitizer polyaniline (PANI) and the nitric oxide (NO) donor S-nitrosoglutathione (GSNO). The system enables mild photothermal therapy (MPTT)–NO synergy via NIR-triggered photothermal heating and controlled NO release. Under NIR irradiation, the generated mild heat induces AF cells to upregulate heat shock proteins (HSPs), thereby enhancing tissue repair. Furthermore, leveraging the thermosensitive release of NO from GSNO, PVA-CS-PANI-GSNO achieves spatiotemporal NO delivery under NIR control, which acts synergistically with MPTT to suppress inflammatory cytokine expression, promote ECM remodeling, and inhibit apoptosis of AF cells, ultimately facilitates the repair of the AF. By overcoming the limitations of systemic anti-inflammatory therapies imposed by the avascular nature of the AF, this strategy offers a promising avenue for the treatment of AF injuries associated with IVDD.
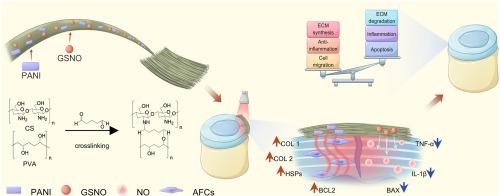
光热触发的no释放纳米纤维膜通过炎症抑制和基质稳定减轻椎间盘退变
椎间盘相关性腰痛是慢性疼痛的主要来源。由于椎间盘退变(IVDD)中的炎症微环境和细胞外基质(ECM)合成受损,纤维环(AF)损伤的愈合能力有限。因此,有效的房颤修复需要调节炎症状态、促进ECM沉积和增强细胞迁移。本研究以静电纺丝生物相容性壳聚糖(CS)和聚乙烯醇(PVA)为原料,结合光敏剂聚苯胺(PANI)和一氧化氮(NO)供体s -亚硝基谷胱甘肽(GSNO),制备了一种多功能光热纳米纤维膜体系。该系统通过nir触发光热加热和控制NO释放,实现轻度光热疗法(MPTT) -无协同作用。在近红外照射下,产生的轻度热诱导AF细胞上调热休克蛋白(HSPs),从而增强组织修复。此外,PVA-CS-PANI-GSNO利用GSNO的热敏性释放NO,在近红外控制下实现时空NO递送,与MPTT协同作用,抑制炎症细胞因子表达,促进ECM重塑,抑制房颤细胞凋亡,最终促进房颤修复。这一策略为治疗与IVDD相关的房颤损伤提供了一条有希望的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


