Distinctive fish collagen drives vascular regeneration by polarizing macrophages to M2 phenotype via TNF-α/NF-κB pathway
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Collagen is a structural protein that plays a critical role in tissue regeneration and is widely utilized in biomedical applications. Recent studies have demonstrated that collagen can modulate macrophage polarization; however, most studies have focused on mammalian collagen such as type I collagen derived from bovine and pig sources. In this study, we performed a comprehensive investigation of the role of collagen derived from aquatic sources, specifically fish swim bladder-derived collagen (SCC), in modulating macrophage inflammation using in vitro and in vivo experiments. First, collagen-coated and collagen-incorporated electrospun poly(ε-caprolactone) (PCL) films were prepared. RNA-Seq analysis showed that SCC could promote M0 and M1 phenotype macrophage transition into M2 through the activation of TNF-α/NF-κB and the downstream signaling pathways. Subcutaneous implantation and artery replacement were also performed. Moreover, SCC prolonged coagulation and synergistically reduces the risk of stenosis. Finally, mouse carotid artery replacement demonstrated that the SCC-modified vascular graft exhibited higher patency in combination with rapid endothelialization and reduced inflammatory responses in vivo. Taken together, we provided strong evidence that fish swim bladder-derived collagen has the capability to modulate macrophage polarization and shows great potential for tissue remodeling and regeneration.
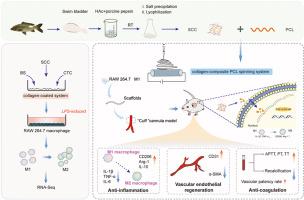
独特的鱼类胶原蛋白通过TNF-α/NF-κB途径将巨噬细胞极化为M2表型,从而驱动血管再生
胶原蛋白是一种结构蛋白,在组织再生中起着至关重要的作用,在生物医学中有着广泛的应用。最近的研究表明,胶原蛋白可以调节巨噬细胞的极化;然而,大多数研究都集中在哺乳动物胶原蛋白上,如从牛和猪中提取的I型胶原蛋白。在这项研究中,我们通过体外和体内实验,全面研究了水生来源的胶原蛋白,特别是鱼鳔来源的胶原蛋白(SCC)在调节巨噬细胞炎症中的作用。首先,制备了胶原包被和胶原包被的电纺丝聚(ε-己内酯)(PCL)薄膜。RNA-Seq分析显示,SCC可通过激活TNF-α/NF-κB及其下游信号通路,促进M0、M1型巨噬细胞向M2型转化。并行皮下植入术和动脉置换术。此外,SCC延长凝血时间并协同降低狭窄的风险。最后,小鼠颈动脉置换实验表明,scc修饰的血管移植物在体内具有更高的通畅性,并能快速内皮化和减少炎症反应。综上所述,我们提供了强有力的证据,证明鱼鳔来源的胶原蛋白具有调节巨噬细胞极化的能力,并显示出巨大的组织重塑和再生潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


