Comparative evaluation of functionalized magnetic perlite–MWCNT composites for removing azacitidine and pemetrexed from water
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
This study evaluates the adsorption capacities and removal efficiencies of four adsorbents, magnetic perlite–Fe₂O₃, perlite–MWCNT, perlite–MWCNT–COOH, and perlite–MWCNT–melamine, for removing pharmaceutical contaminants azacitidine (AZA) and pemetrexed (PMX) from aqueous solutions. The magnetic perlite–MWCNT composite was synthesized via chemical vapor deposition, and the functionalized MWCNTs were prepared by controlled acid oxidation. Perlite–MWCNT–melamine demonstrated the maximum adsorption capacities (Qm), achieving 570 mg·g−1 for AZA and 630 mg·g−1 for PMX. Using Box–Behnken response surface methodology (RSM), we optimized contact time, adsorbent dosage, and pH to maximize removal efficiency. The adsorption process followed the Langmuir isotherm and pseudo-second-order kinetics, indicating monolayer chemisorption. Thermodynamic analysis confirmed that adsorption was spontaneous and endothermic. Additionally, all adsorbents demonstrated excellent reusability over six cycles, with minimal loss in removal efficiency. These findings suggest that melamine-functionalized perlite–MWCNT composites are highly effective and durable adsorbents for practical pharmaceutical contaminant removal in water treatment applications.
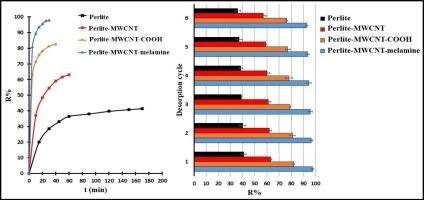
功能化磁性珍珠岩- mwcnt复合材料去除水中氮扎胞苷和培美曲塞的比较评价
研究了磁性珍珠岩- fe₂O₃、珍珠岩- mwcnt、珍珠岩- mwcnt - cooh和珍珠岩- mwcnt -三聚氰胺四种吸附剂对水中药物污染物阿扎胞苷(AZA)和培美曲塞(PMX)的吸附能力和去除效率。采用化学气相沉积法制备磁性珍珠岩- mwcnt复合材料,并采用可控酸氧化法制备功能化mwcnt。珍珠岩- mwcnts -三聚氰胺表现出最大的吸附量(Qm),对AZA的吸附量为570 mg·g−1,对PMX的吸附量为630 mg·g−1。采用Box-Behnken响应面法(RSM)对接触时间、吸附剂投加量和pH进行优化,以达到最大的去除效果。吸附过程遵循Langmuir等温线和拟二级动力学,为单层化学吸附。热力学分析证实吸附是自发的吸热吸附。此外,所有吸附剂在6个循环中表现出良好的可重复使用性,去除效率损失最小。这些发现表明,三聚氰胺功能化珍珠岩- mwcnt复合材料是一种高效耐用的吸附剂,可用于水处理中实际的药物污染物去除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


